Abstract
In the outside world, response selection often requires the processing of information from different spatial dimensions. Yet, most neuroscientific approaches to the topic only employ variations in one dimension, namely the horizontal left-right axis. Hence, virtually nothing is known about the neuronal mechanisms underlying response selection in more than one dimension. We investigated this aspect with the help of a two-dimensional flanker task using EEG and source localization techniques. The data shows that response selection processes are differentially modulated across different dimensions. However, this modulation is restricted to conditions imposing increased demands on response selection. In such situations, a distributed fronto-parietal network mediates intensified conflict monitoring processes as well as response inhibition processes. In case response selection is carried out in the horizontal dimension, those brain areas are more active than during response selection in the vertical dimension. Attentional selection processes were not affected. The study's findings are of relevance to our understanding to everyday functioning where response selection is usually carried out in two or three dimensions and not a single dimension as usually investigated in cognitive neuroscience.
Similar content being viewed by others
Introduction
The investigation of neuronal mechanisms underlying response selection has a long tradition in cognitive neuroscience and is of considerable practical importance. However, classical paradigms like Flanker or Simon tasks usually use uni-dimensional stimulus arrays in a left-right dimension to examine neuronal processes of response selection. Hence, virtually nothing is known about the neuronal mechanisms that underlie response selection in more than one dimension.
Results from cognitive psychology suggest that spatial dimensions differentially affect psychomotor processes1,2,3,4,5. Here, the so called right-left prevalence effect has consistently been reported6. This effect refers to the finding that psychomotor processes are faster in the horizontal dimension than in the vertical dimension. It depends on a variety of factors including stimulus-response configurations (left/right positioning of responding hands) and the relative saliency of stimuli in the different dimensions1,3. Though the nature of the right-left prevalence is a matter of debate3, findings by Rubichi et al.4 suggest that this effect persists even when stimulus-response sets do not bias a particular spatial dimension. As compared to the ‘vertical dimension’, the ‘horizontal dimension’ generally seems to be processed differently. Against this background, we hypothesize that neuronal processes underlying response selection processes differ between the horizontal and vertical dimensions. It has been suggested that the right-left prevalence effect emerges because in everyday life, right-left discriminations are more practiced than top-down discriminations4. This practice leads to a high degree of automation. This automation aspect is of importance when considering response and conflict monitoring processes. Concerning ‘automation’, the conflict monitoring account of cognitive control suggests that conflict should emerge from the requirement to override a pre-potent response because this results in competition between the desired response and the previous one that needs to be overridden7,8. We therefore hypothesize that especially conflict monitoring processes shall be differentially affected by dimensions of space.
We therefore chose to examine response selection processes with a flanker task where not only responses in the left-right dimension were required, but in the vertical or far-near axis as well while applying EEG-measures in combination with source localization techniques. What is typically found in the flanker task is that response selection and cognitive control processes are intensified in incompatible stimulus-response mappings (i.e. reaction times and error rates increase) where the flanker arrows point to the opposite direction of the target arrow7,9. These conditions have been suggested to induce a conflict between response tendencies8. These conflicts have been shown to be reflected by the N2 event-related potential (ERP) and it has been shown that activation differences in the anterior cingulate cortex (ACC) contribute to the modulation of the N29,10,11,12,13. Given that the ‘horizontal dimension’ is highly automatized4, we hypothesize that conflict monitoring processes are strong in the horizontal dimension. This should be reflected by larger compatibility effects in RTs in the horizontal dimension as compared to the vertical dimension. In line with that, conflict processing in the horizontal dimension should elicit a larger N2 and hence increased activity of the ACC than in the vertical dimension. As the spatial dimension is supposed to modulate conflict monitoring processes, inferior parietal structures, which are important for the processing of spatial codes14, may also be involved. Furthermore, previous results suggest that during the resolution of response conflict, inhibitory processes also play an important role15,16,17. In this regard, Chmielewski et al.15 showed that a positive, P3-like deflection that is associated with brain activity changes in networks mediating response inhibition can be seen at fronto-central electrode sites. For the current study, we suggest that in case of response conflict, both conflict monitoring (N2) and response inhibition processes should also be enhanced in the horizontal dimension. Aside from these correlates of processes occurring at the response selection level, we will also analyze attentional selection processes to examine whether the spatial dimension only affects cognitive control processes at the response selection level, or also at the stimulus processing level.
Results
Behavioral data
The rate of correct responses and the mean reaction times (RTs) were analyzed using repeated measures ANOVAs. For descriptive statistics the mean and standard error of the mean (s.e.m.) are given. For RTs, the main effect “compatibility” (F(1,23) = 304.79; p < .001; η2 = .930) revealed that RTs were longer in the incompatible condition (633 ms ± 11) than in the compatible condition (518 ms ± 10). The main effect “dimension” was not significant (F(1,23) = 0.76; p > .3), but there was an interaction of “compatibility × dimension” (F(1,23) = 85.99; p < .001; η2 = .789) showing that compatibility effects are differentially strong in the two dimensions. To further analyze this, we calculated a measure for the degree of response conflict by subtracting RTs in the compatible condition from RTs in the incompatible condition. This was done separately for the horizontal and vertical dimension. The obtained compatibility effect measure was significantly different (t23 = 9.27; p < .001) for the horizontal (128 ms ± 0.6) and vertical (100 ms ± 0.7) dimensions, thus showing that dimension modulates compatibility effects.
For the percentage of correct responses, there was a main effect of “compatibility” (F(1,23) = 21.32; p < .001; η2 = .481) showing that the rate of correct responses was lower in the incompatible condition (80% ± 0.4) than in the compatible condition (86.6% ± 0.5). There was no main effect of “dimension” or an interaction of “compatibility × dimension” (all F < 0.11; p > .7). With respect to the RT data, the results hence show that there was no speed-accuracy trade-off.
However, it may be argued that in the experimental setup (refer methods section) the vertical dimension is confounded with a movement in the depth plane and hence the near-far dimension. We therefore conducted a behavioural supplemental experiment (refer supplementary material) controlling for this potential confound. This experiment shows that when controlling for this confound the effects were still the same; i.e. the compatibility effect was larger in the horizontal than in the vertical dimension. The size of effects was similar as in the above described data from the main experiment. The near-far confound is therefore not critical for the results pattern.
Neurophysiological data
For the main experiment EEG data was collected. The P1 and N1 components reflecting attentional processing18,19 evoked by the flanker and target stimuli are shown in Figure 1. The electrodes used for the statistical analysis of experimental effects reflect the maximum activity, which was statistically validated as outlined in the methods section.
Event-related current source densities depicting the P1 and N1 to flanker and target stimuli at electrodes P7 and P8 for the different experimental conditions.
Black lines denote the compatible horizontal condition, brown lines denote the compatible vertical condition, green lines the incompatible horizontal condition and red lines the incompatible vertical condition. The y-axis is given in μV/m2 and the x-axis shows the time in ms. The onsets of the flanker and target stimuli are indicated by arrows in the bottom part of the illustration. The baseline was set to immediately precede the presentation of the flanker stimulus (as illustrated by the black bar from −300 to −200 ms prior to target onset/time point 0). The scalp topography plots show the topographical distribution of the P1 and N1 components in incompatible trials.
For the flanker P1 and N1, there were generally no effects, neither for amplitude nor for latency (all F < 0.7; p > .3). For the target P1, there was a main effect of “compatibility” (F(1,23) = 26.87; p < .001; η2 = .539) showing that the P1 was larger in the incompatible (28.88 μV/m2 ± 3.2) than in the compatible condition (24.2 μV/m2 ± 2.6). The main effect of “electrode” (F(1,23) = 12.99; p = .001; η2 = .361) showed that the P1 was larger at electrode P8 (31.4 μV/m2 ± 3.4) than at P7 (21.7 μV/m2 ± 3). Likewise, there was a main effect of “compatibility” for the target N1 (F(1,23) = 19.78; p < .001; η2 = .462) showing the N1 was larger (i.e., more negative) in incompatible (−13.91 μV/m2 ± 2.2) than in compatible trials (−8.3 μV/m2 ± 1.3). The main effect of “electrode” (F(1,23) = 4.63; p = .042; η2 = .168) showed that the N1 was larger at electrode P8 (−12.77 μV/m2 ± 1.8) than at electrode P7 (−9.94 μV/m2 ± 1.9). No other effects were significant for the target P1 and N1 amplitudes (all F < 0.6; p > .5) and there were no latency effects (all F < 0.9; p > .3). Taken together, these data show that the manipulation of vertical vs. horizontal dimensions had no effect on perceptual and attentional processes.
The N2 and P3-like components at electrode FCz as well as the associated scalp topography maps are shown in Figure 2. For the N2, the repeated measures ANOVA revealed a main effect of “compatibility” (F(1,23) = 70.90; p < .001; η2 = .755) showing that the N2 was larger (i.e., more negative) in incompatible (−25.74 μV/m2 ± 1.62) than in compatible trials (−10.88 μV/m2 ± 0.57). The main effect of “dimension” (F(1,23) = 7.74; p = .011; η2 = .252) showed that the N2 was larger in the horizontal (−20.82 μV/m2 ± 1.54) than in the vertical dimension (−15.77 μV/m2 ± 0.82). Importantly, there was an interaction of “compatibility × dimension” (F(1,23) = 8.98; p = .006; η2 = .281). Bonferroni-corrected post-hoc paired t-tests revealed that there was no difference in N2 amplitudes between the vertical (−9.99 μV/m2 ± 0.96) and horizontal dimensions (−11.72 μV/m2 ± 0.85) in compatible trials (t23 = −0.98; p > .3). However, the N2 was larger in the horizontal (−29.93 μV/m2 ± 2.66) than in the vertical dimension (−21.55 μV/m2 ± 1.32) in incompatible trials (t23 = −3.14; p = .002). Regarding N2 latencies, there were generally no effects (all F < 1.1; p > .2). The sLORETA analysis contrasting the incompatible horizontal condition against the incompatible vertical condition suggested that the observed N2 amplitude differences are due to activity differences in the medial frontal gyrus (BA9), anterior cingulate cortex (ACC) (BA24) and the posterior medial frontal gyrus (BA6) including the supplementary motor area (SMA) as well as the right inferior parietal cortex (rIPC) (refer Figure 2). As a ‘sanity check’ for the reliability of the sLORETA results, we also contrasted the compatible against the incompatible condition (incompatible > compatible). This contrast revealed activation differences in the dorsal ACC (BA24) (refer supplementary Figure 1) which is well in line with previously reported findings (e.g. Folstein & Van Petten, 2008; van Veen & Carter, 2002). This underlines that the sources provided for the other results are reliable.
Event-related current source densities depicting the N2 and P3-like components at electrode FCz for the different experimental conditions.
Black lines denote the compatible horizontal condition, brown lines denote the compatible vertical condition, green lines the incompatible horizontal condition and red lines the incompatible vertical condition. The y-axis is given in μV/m2 and the x-axis shows the time in ms. The onsets of the flanker and target stimuli are indicated by arrows above the ERP curves. The baseline was set to immediately precede the presentation of the flanker stimulus (as illustrated by the black bar from −300 to −200 ms prior to target onset/time point 0). The scalp topography plots (top right) show the topographical distribution of the N2 and P3-like components (separately for the compatible vs. incompatible trials and horizontal vs. vertical task conditions). The sLORETA plots (bottom and top) depict the source of the difference between the horizontal and the vertical task conditions in incompatible trials.
As can be seen in Figure 2, the P3-like component seems to be larger in the incompatible horizontal condition than in the incompatible vertical condition. This becomes especially obvious when considering peak-to-peak amplitudes. Analyzing the P3-like component using peak-to-peak amplitudes in a repeated measures ANOVA revealed a main effect of “compatibility” (F(1,23) = 136.26; p < .001; η2 = .856) showing that the P3-like component was larger in the incompatible (15.38 μV/m2 ± 1.28) than in the compatible condition (47.42 μV/m2 ± 2.61). The main effect of “dimension” (F(1,23) = 9.70; p = .005; η2 = .297) showed that the P3-like component was larger in the horizontal (35.29 μV/m2 ± 2.31) than in the vertical dimension (27.56 μV/m2 ± 1.53). Again, there was an interaction of “compatibility × dimension” (F(1,23) = 13.59; p = .001; η2 = .371). Bonferroni-corrected post-hoc paired t-tests revealed that there was no difference between the vertical (16.97 μV/m2 ± 2.14) and horizontal dimensions (13.78 μV/m2 ± 1.56) in compatible trials (t23 = −1.05; p > .2). Yet, the P3-like component was larger in the horizontal (56.69 μV/m2 ± 4.06) than in the vertical dimension (38.14 μV/m2 ± 2.83) in incompatible trials (t23 = −3.14; p = .002). Contrasting the incompatible vertical and the incompatible horizontal conditions with sLORETA revealed activation differences in the right middle frontal gyrus (refer Figure 2).
Discussion
In the current study, we examined the neuronal mechanisms underlying response selection and conflict monitoring processes in multidimensional space. Currently, virtually nothing is known as to whether these important executive control functions differ across dimensions of space.
Our results show that response selection processes are differentially modulated as a function of the horizontal and vertical as well as vertical/depth dimensions and by the degree of conflict during response selection. In the non-conflicting compatible condition, no differences in behavioral parameters were evident between the spatial dimensions. Yet, the degree of response conflict was larger in incompatible trials when response selection was carried out in the horizontal dimension as compared to the vertical dimension. Because the experimental setup controlled for this, this result was unbiased with respect to a number of different factors that have been shown to modulate right-left prevalence effects (i.e. positioning of the responding hand, saliency of stimuli and instructions favoring one dimension). The supplementary experiment shows that though in the main experiment movements in the vertical dimension also involve a movement in the depth plane (near-far dimension), the latter aspect does not drive the effects obtained. It is the vertical vs. the horizontal dimension that affects response selection processes.
Given that the P1 and N1 ERPs were not differentially modulated by the horizontal or vertical dimension, the psychophysiological data suggest that this effect does not hark back to differences in perceptual or attentional processing. Instead, the psychophysiological data shows that response selection processes were differentially modulated as a function of dimension in space and the degree of conflict during response selection. The N2, which is a correlate of conflict monitoring and response selection processes12, was larger in the horizontal than in the vertical dimension in (incompatible) conflict trials, but not in compatible trials. This differential modulation of the N2 in the incompatible condition was related to activity changes in the medial frontal gyrus (BA9), anterior cingulate cortex (ACC) (BA24) and the posterior medial frontal gyrus (BA6) including the supplementary motor area (SMA) as well as the right inferior parietal cortex (rIPC). The ACC (BA24) is well-known to modulate the N2 component in conflicting tasks7,9,12 and similar findings were obtained for the supplementary motor area20,21,22. Both of these areas have been suggested to mediate the selection of actions and changes of voluntary action plans during conflict. This does however not apply to the rIPC, which is usually not activated in conflict monitoring tasks. The observed rlPC activation differences are therefore most likely due to the differences in spatial dimensions between experimental conditions which are coded by this area14.
Aside from the N2, a P3-like component, which has been suggested to reflect response inhibition processes15, was also larger during response selection in the horizontal as compared to the vertical dimension. Source localization suggests that activity differences in the right middle frontal gyrus contribute to this modulation. The right middle frontal gyrus has been shown to be involved in response inhibition processes23,24,25. Previous results suggest that response inhibition processes contribute to conflict resolution mechanisms by inhibiting the unwanted (incompatible) response representation15,16,26. Against this background, it seems that these processes are also intensified in the horizontal dimension. This underlines that cognitive control processes are not uniformly modulated by the spatial dimensions in which response selection needs to be carried out.
Overall, the behavioral as well as the psychophysiological data show that the spatial dimension has an effect on response monitoring processes only when there is a conflict between response alternatives. It has been argued that we continuously evaluate the level of conflict between the available response alternatives7,8: In the case of a conflict, the monitoring system initiates cognitive control processes to bias conflict resolution towards the instruction-appropriate response. Logically following from this, cognitive resources are less strained when there is no conflict. In line with this, the results suggest the spatial dimension in which response selection is carried out only modulates response selection when cognitive resources are stressed. Our data shows that the modulation of the degree of cognitive conflict follows known right-left prevalence effects of spatial dimensions (i.e. the degree of conflict is higher in the more prevalent horizontal dimension). Even though there is still no well-justified logical explanation for the existence of right-left prevalence effects, it has been suggested that this effect emerges because (i) everyday life right-left discriminations are more practiced than top-down discriminations and also (ii) because top-down discriminations may require more effort4. With regard to the first aspect (automation), the conflict monitoring account of cognitive control suggests that conflict emerges from the necessity to override a pre-potent response which ultimately results in competition between the desired response and the one being overridden7,8. Against this background, it seems reasonable that conflict monitoring processes are enhanced in the horizontal dimension. The second aspect is also directly supported by our data, showing that the degree of conflict is larger in the horizontal dimension. This is also underlined by the supplementary experiment.
A possible alternative explanation of the current findings and related to the automation aspect, may be related to a cultural ‘scanning bias’. In western cultures the left-right dimension may be particularly prominent due to reading habits. In this regard it may be interesting to examine how the dimensional modulations come across in eastern populations show a vertical reading direction. It is possible that the results are reversed. On a related point it may be interesting to independently vary the congruency of the vertical and horizontal distractor stimuli. This would result in fully congruent trials (all arrows point in the same direction), fully incongruent trials (all distractors point in the opposite direction of the target) and partial incongruent trials (half of the distractors are congruent, the others are incongruent). This may be combined with an in-depth analysis of neural oscillations. As can be seen in Figure 2, N2 and P3-like components may be driven by a common oscillatory response. Beamforming approaches may examine whether this oscillation is related to the mid-cingulate cortex that is known be important in cognitive control.
In summary, we showed that that response selection processes are differentially modulated across dimensions of a two-dimensional space. However, this modulation is restricted to conditions imposing increased demands on response selection. In such situations, a distributed fronto-parietal network mediates intensified conflict monitoring processes as well as response inhibition processes when response selection is carried out in the horizontal dimension as compared to the vertical dimension.
Methods
Participants
In total, 24 participants (male = 13; female = 11) were included in the study. The mean age was 24.18 (±2.52) years and ranged from 20 to 29 years. All participants were right-handed and had no history of psychiatric or neurological diseases. All participants were university students and received financial reimbursement. Each participant gave written informed consent and was treated in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the Technische Universität Dresden.
Task
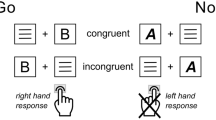
The experimental setup is shown in Figure 3. We used a modified version of a flanker task27,28,29, which was extended by a vertical stimulus-response level in order to produce two stimulus-response dimensions. The equally-sized arrow stimuli were presented vertically and horizontally arranged. The target stimulus was centered on the screen, with the arrowhead either pointing left, right, up or down. The central target stimulus was accompanied by flanker stimuli positioned left, right, below and atop of the target stimulus. All stimuli were presented in white color on a black background. All arrows (i.e., target and flanker arrows) each were2 cm high and wide, which accords to 2° visual angle (at a viewing distance of 56.5 cm). The identical stimulus height and width of the stimuli ensured that no dimension was biased due to perceptual or attentional processes. The edge-to-edge distance between the stimuli was 1.5 cm. The participants were instructed to place equal emphasis on the vertical and the horizontal dimension.
Schematical illustration of the experimental setup (see text for further description).
Participants were presented a dimensional flanker stimulus and were asked to move a joystick into the direction of the centrally presented target stimulus. The flanker stimuli preceded the target by 200 ms to induce conflict in the incompatible flanker-target combination of stimuli. The joystick was centered in the middle of the participant's body.
In case of a compatible trial, both target and flanker stimuli pointed in the same direction (either up, down, left, or right). In incompatible trials, the target and the four flankers pointed into different directions. In order to avoid a possible horizontal stimulus-response configuration bias (evoked by the congruency of arrow direction and hand positioning), a game joystick positioned centrally in front of the participants was used as response device. The participants were instructed to use their right hand to move the stick into the direction the target stimulus was pointing and to refrain from responding to the flanker stimuli. In case of an upwards pointing target, participants were instructed to move the stick forward. If the target pointed downwards the participants were asked to deflect the stick downwards (i.e. towards themselves). For a valid response, the stick had to be deflected by a minimum of 10 degrees out of the vertical or horizontal joystick axes. The target was preceded by the flanker stimuli with a stimulus onset asynchrony (SOA) of 200 ms. The target was displayed for 300 ms. Target and flankers were switched off simultaneously. To administer time pressure, the participants were asked to respond within 1000 ms. In trials without a valid response i.e. if the joystick was not sufficiently deflected or the participants completely missed a response, a beep was presented via headphones (1000 Hz, 150 ms). The mean response-stimulus interval (RSI) was 1100 ms, randomly ranging between 900 and 1300 ms. Participants were instructed to move the joystick back into the initial position after each trial. Six blocks of 120 trials each were presented in the task. In each block, compatible (70%) and incompatible trials (30%) were presented in a pseudo-randomized order11. Target and distractor direction frequency were counterbalanced for compatible and incompatible trials. The order of ‘vertical’ and ‘horizontal’ trials was also pseudo-randomized across trials. The task was practiced prior to the experiment in a training block for approx. 3 minutes.
EEG recording and analysis
The EEG was recorded from 60 Ag/AgCl electrodes arranged in equidistant positions. The ground and reference electrode were placed at coordinates theta = 58, phi = 78 and theta = 90, phi = 90, respectively. The sampling rate was 500 Hz. All electrode impedances were kept below 5 kΩ. Data processing involved a manual inspection of the data to remove technical artifacts. After manual inspection, a band-pass filter ranging from 0.5 to 40 Hz (48 db/oct) was applied and data was down-sampled to 256 Hz. After filtering, the raw data were inspected a second time. To correct recurring artifacts (horizontal and vertical eye movements and blinks as well as pulse actifacts) an independent component analysis (ICA; Infomax algorithm) was applied to the un-epoched data set. Components that reveal horizontal and vertical eye movements, blinks and pulse artifacts were visually identified by means of recurrent (and potentially periodically appearing) similar waveform and by means of scalp topography. ICA components reflecting the above mentioned artifacts were then discarded. Afterwards, the EEG was segmented into epochs of 1500 ms length. Time point 0 was set to the onset of target stimulus presentation. Each epoch started 500 ms before and ended 1000 ms after the locking point. Only trials with correct reactions were included in this analysis. After epoching, an automated artifact rejection procedure was applied, using a maximal value difference of 200 μV in a 100 ms interval as well as an activity below 0.5 μV in a 200 ms period as rejection criteria. Afterwards, a current source density (CSD) transformation30 was applied to re-reference the data. After this, the resulting CSD values were stated in μV/m2. The CSD transformation was used in order to eliminate the reference potential from the data. Another advantage of the CSD-transformation is that it works as a spatial filter30, making it possible to identify electrodes that best reflect activity related to cognitive processes. For baseline correction, a 100 ms time interval between −300 and −200 ms before target presentation was used. The baseline was thus set just before the presentation of the flanker stimuli. Finally, averages were calculated across all trials for each condition at the single subject level. Because segments were separately averaged for compatible vs. incompatible trials and for the horizontal vs. vertical dimension, averaging yielded four conditions for each subject. Electrode sites used for ERP data quantification were first chosen on the basis of visual inspection of the scalp topographies and then verified using a statistical approach. In this statistical verification approach, we compared each electrode against an average of all other electrodes using Bonferroni-correction for multiple comparisons (critical threshold p = .0007; i.e. significance level p = .05 divided by the number of electrodes under bonferroni correction). Only electrodes that showed significantly larger mean amplitudes (i.e., negative for N-potentials and positive for the P-potentials) than the remaining electrodes were chosen31. In accordance with the obtained results, amplitude and latency of the N2 and the P3-like components were quantified at electrode FCz on a single-subject level. Latency and amplitude of the visual P1 and N1 following flanker and target stimuli were quantified at electrode P7 and P8 on a single-subject level.
Source localization (sLORETA)
Source localization was carried out using sLORETA (standardized low resolution brain electromagnetic tomography32). sLORETA gives a single linear solution to the inverse problem based on extra-cranial measurements32,33,34. For sLORETA, the intracerebral volume is partitioned in 6239 voxels with 5 mm spatial resolution. The standardized current density at each voxel is calculated in a realistic head model35 using the MNI152 template. The voxel-based sLORETA-images were then compared between trials using the sLORETA-built-in voxel-wise randomization tests with 2000 permutations based on statistical nonparametric mapping. Single subject data were used as the basis for the sLORETA analysis. Voxels with significant differences (p < .01, corrected for multiple comparisons) between test sessions were located in the MNI-brain and Brodmann areas (BAs). Additionally, coordinates in the MNI-brain were determined using the sLORETA software www.unizh.ch/keyinst/NewLORETA/sLORETA/sLORETA.htm. Results obtained with sLORETA have been shown to reveal high convergence with results from fMRI data. Furthermore, it has been mathematically proven that the used algorithm provides reliable results without localization bias34.
Statistical analysis
The data was analyzed using repeated measures ANOVAs. For the behavioral data, the factors “compatibility” (compatible vs. incompatible) and “dimension” (horizontal vs. vertical) were used. For the neurophysiological data, the factor “electrode” was included wherever necessary (P1 and N1 data). Prior to data analyses, all variables were checked for normal distribution using Kolmogorov-Smirnov tests. These indicated that all variables entered into the analysis were normally distributed (all z < 0.9; p > .3). Post-hoc tests were Bonferroni-corrected.
References
Vu, K.-P. L., Minakata, K. & Ngo, M. K. Influence of auditory and audiovisual stimuli on the right-left prevalence effect. Psychol. Res. 78, 400–410 (2014).
Proctor, R. W., Koch, I., Vu, K.-P. L. & Yamaguchi, M. Influence of display type and cue format on task-cuing effects: dissociating switch cost and right-left prevalence effects. Mem. Cognit. 36, 998–1012 (2008).
Rubichi, S., Vu, K.-P. L., Nicoletti, R. & Proctor, R. W. Spatial coding in two dimensions. Psychon. Bull. Rev. 13, 201–216 (2006).
Rubichi, S., Nicoletti, R. & Umiltà, C. Right-left prevalence with task-irrelevant spatial codes. Psychol. Res. 69, 167–178 (2005).
Hommel, B. No prevalence of right-left over top-bottom spatial codes. Percept. Psychophys. 58, 102–110 (1996).
Nicoletti, R. & Umiltà, C. Right-left prevalence in spatial compatibility. Percept. Psychophys. 35, 333–343 (1984).
Botvinick, M. M., Cohen, J. D. & Carter, C. S. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8, 539–546 (2004).
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S. & Cohen, J. D. Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652 (2001).
Van Veen, V. & Carter, C. S. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol. Behav. 77, 477–482 (2002).
Beste, C. et al. Mechanisms mediating parallel action monitoring in fronto-striatal circuits. NeuroImage 62, 137–146 (2012).
Beste, C., Domschke, K., Falkenstein, M. & Konrad, C. Differential modulations of response control processes by 5-HT1A gene variation. NeuroImage 50, 764–771 (2010).
Folstein, J. R., Van Petten, C. & Rose, S. A. Novelty and conflict in the categorization of complex stimuli. Psychophysiology 45, 467–479 (2008).
Willemssen, R., Falkenstein, M., Schwarz, M., Müller, T. & Beste, C. Effects of aging, Parkinson's disease and dopaminergic medication on response selection and control. Neurobiol. Aging 32, 327–335 (2011).
Husain, M. & Nachev, P. Space and the parietal cortex. Trends Cogn. Sci. 11, 30–36 (2007).
Chmielewski, W. X., Mückschel, M., Roessner, V. & Beste, C. Expectancy effects during response selection modulate attentional selection and inhibitory control networks. Behav. Brain Res. 274, 53–61 (2014).
Klein, P.-A., Petitjean, C., Olivier, E. & Duque, J. Top-down suppression of incompatible motor activations during response selection under conflict. NeuroImage 86, 138–149 (2014).
Stürmer, B., Siggelkow, S., Dengler, R. & Leuthold, H. Response priming in the Simon paradigm. A transcranial magnetic stimulation study. Exp. Brain Res. 135, 353–359 (2000).
Wascher, E. & Beste, C. Tuning perceptual competition. J. Neurophysiol. 103, 1057–1065 (2010).
Herrmann, C. S. & Knight, R. T. Mechanisms of human attention: event-related potentials and oscillations. Neurosci. Biobehav. Rev. 25, 465–476 (2001).
Mayer, A. R. et al. Modeling conflict and error in the medial frontal cortex. Hum. Brain Mapp. 33, 2843–2855 (2012).
Nachev, P., Rees, G., Parton, A., Kennard, C. & Husain, M. Volition and conflict in human medial frontal cortex. Curr. Biol. CB 15, 122–128 (2005).
Rushworth, M. F. S., Walton, M. E., Kennerley, S. W. & Bannerman, D. M. Action sets and decisions in the medial frontal cortex. Trends Cogn. Sci. 8, 410–417 (2004).
Dambacher, F. et al. The role of right prefrontal and medial cortex in response inhibition: interfering with action restraint and action cancellation using transcranial magnetic brain stimulation. J. Cogn. Neurosci. 26, 1775–1784 (2014).
Dambacher, F. et al. A network approach to response inhibition: dissociating functional connectivity of neural components involved in action restraint and action cancellation. Eur. J. Neurosci. 39, 821–831 (2014).
Drueke, B. et al. The role of 5-HT in response inhibition and re-engagement. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 23, 830–841 (2013).
Verleger, R., Kuniecki, M., Möller, F., Fritzmannova, M. & Siebner, H. R. On how the motor cortices resolve an inter-hemispheric response conflict: an event-related EEG potential-guided TMS study of the flankers task. Eur. J. Neurosci. 30, 318–326 (2009).
Kopp, B., Rist, F. & Mattler, U. N200 in the flanker task as a neurobehavioral tool for investigating executive control. Psychophysiology 33, 282–294 (1996).
Beste, C., Saft, C., Andrich, J., Gold, R. & Falkenstein, M. Error processing in Huntington's disease. PloS One 1, e86 (2006).
Willemssen, R., Müller, T., Schwarz, M., Falkenstein, M. & Beste, C. Response monitoring in de novo patients with Parkinson's disease. PloS One 4, e4898 (2009).
Nunez, P. L. & Pilgreen, K. L. The spline-Laplacian in clinical neurophysiology: a method to improve EEG spatial resolution. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 8, 397–413 (1991).
Mückschel, M., Stock, A.-K. & Beste, C. Psychophysiological mechanisms of interindividual differences in goal activation modes during action cascading. Cereb. Cortex N. Y. N 1991 24, 2120–2129 (2014).
Pascual-Marqui, R. D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24 Suppl D 5–12 (2002).
Marco-Pallarés, J., Grau, C. & Ruffini, G. Combined ICA-LORETA analysis of mismatch negativity. NeuroImage 25, 471–477 (2005).
Sekihara, K., Sahani, M. & Nagarajan, S. S. Localization bias and spatial resolution of adaptive and non-adaptive spatial filters for MEG source reconstruction. NeuroImage 25, 1056–1067 (2005).
Fuchs, M., Kastner, J., Wagner, M., Hawes, S. & Ebersole, J. S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 113, 702–712 (2002).
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG), BE4045/10-1 and BE4045/10-2.
Author information
Authors and Affiliations
Contributions
M.M. and C.B. designed the experiment, collected data, analyzed the data and wrote the manuscript.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary information
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
About this article
Cite this article
Mückschel, M., Beste, C. Psychophysiological mechanisms underlying response selection in multidimensional space. Sci Rep 5, 7759 (2015). https://doi.org/10.1038/srep07759
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep07759
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.