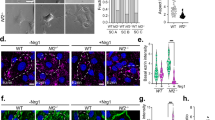
Abstract
Mutations in the neurofibromatosis type 2 (NF2) gene cause formation of schwannomas and other tumors in the nervous system. The NF2 protein, Schwannomin/Merlin, is a cytoskeleton-associated tumor suppressor regulated by phosphorylation at serine 518 (S518). Unphosphorylated Schwannomin restricts cell proliferation in part by inhibiting Rac- and p21-activated kinase (Pak). In a negative-feedback loop, Pak phosphorylates Schwannomin inactivating its ability to inhibit Pak. Little is known about receptor mechanisms that promote Pak activity and Schwannomin phosphorylation. Here we demonstrate in primary Schwann cells (SCs) that Schwannomin is rapidly phosphorylated on S518 by Pak following laminin-1 binding to β1 integrin, and by protein kinase A following neuregulin-1β (NRG1β) binding to ErbB2/ErbB3 receptors. These receptors, together with phosphorylated Schwannomin, P-Pak, Cdc42 and paxillin are enriched at the distal tips of SC processes, and can be isolated as a complex using β1 integrin antibody. Dual stimulation with laminin-1 and NRG1β does not synergistically increase Schwannomin phosphorylation because ErbB2 kinase partially antagonizes integrin-dependent activation of Pak. These results identify two parallel, but interactive pathways that inactivate the tumor suppressor activity of Schwannomin to allow proliferation of subconfluent SCs. Moreover, they identify ErbB2, ErbB3 and β1 integrins as potential therapeutic targets for NF2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Adam L, Vadlamudi R, Kondapaka SB, Chernoff J, Mendelsohn J, Kumar R . (1998). Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J Biol Chem 273: 2823.
Alfthan K, Heiska L, Gronholm M, Renkema GH, Carpen O . (2004). Cyclic AMP-dependent protein kinase phosphorylates merlin at serine 518 independently of p21-activated kinase and promotes Merlin-Ezrin heterodimerization. J Biol Chem 279: 18559–18566.
Brown MC, West KA, Turner CE . (2002). Paxillin-dependent paxillin kinase linker and p21-activated kinase localization to focal adhesions involves a multistep activation pathway. Mol Biol Cell 13: 1550–1565.
Chen LM, Bailey D, Fernandez-Valle C . (2000). Association of beta 1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J Neurosci 20: 3776–3784.
Chen ZL, Strickland S . (2003). Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol 163: 889–899.
Chernousov MA, Carey DJ . (2000). Schwann cell extracellular matrix molecules and their receptors. Histol Histopathol 15: 593–601.
Coles LC, Shaw PE . (2002). PAK1 primes MEK1 for phosphorylation by Raf-1 kinase during cross-cascade activation of the ERK pathway. Oncogene 21: 2236–2244.
Del Pozo MA, Price LS, Alderson NB, Ren X-D, Schwartz MA . (2000). Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J 19: 2008–2014.
Dubovy P, Svizenska I, Jancalek R, Klusakova I, Houstava L, Haninec P et al. (1999). Immunohistochemical localization of laminin-1 in the acellular nerve grafts is associated with migrating Schwann cells which display corresponding integrin receptors. Gen Physiol Biophys 18: 63–65.
Fernandez-Valle C, Gwynn L, Wood PM, Carbonetto S, Bunge MB . (1994). Anti-beta 1 integrin antibody inhibits Schwann cell myelination. J Neurobiol 25: 1207–1226.
Fernandez-Valle C, Tang Y, Ricard J, Rodenas-Ruano A, Taylor A, Hackler E et al. (2002). Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet 31: 354–362.
Garratt AN, Britsch S, Birchmeier C . (2000). Neuregulin, a factor with many functions in the life of a Schwann cell. Bioessays 22: 987–996.
Hansen MR, Linthicum FH . (2004). Expression of neuregulin and activation of erbB receptors in vestibular schwannomas: possible autocrine loop stimulation. Otol Neurotol 25: 155–159.
Howe AK, Juliano RL . (2000). Regulation of anchorage-dependent signal transduction by protein kinase A and p21-activated kinase. Nat Cell Biol 2: 593–600.
Iacovelli J, Lopera J, Bott M, Baldwin E, Khaled A, Uddin N et al. (2007). Serum and forskolin cooperate to promote G1 progression in Schwann cells by differentially regulating cyclin D1, E1, and p27(Kip) expression. Glia 55: 1638–1647.
James MF, Manchanda N, Gonzalez-Agosti C, Hartwig JH, Ramesh V . (2001). The neurofibromatosis 2 protein product merlin selectively binds F-actin but not G-actin, and stabilizes the filaments through lateral association. Biochem J 356: 377–386.
Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO . (2003). Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet 12: 1211–1221.
Kim HA, DeClue JE, Ratner N . (1997). cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res 49: 236–247.
Kissil JL, Johnson KC, Eckman MS, Jacks T . (2002). Merlin phosphorylation by p21-activated kinase 2 and effects of phosphorylation on merlin localization. J Biol Chem 277: 10394–10399.
Lee JW, Juliano RL . (2004). Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells 30: 188–202.
Lotti LV, Di Lazzaro C, Zompetta C, Frati L, Torrisi MR . (1992). Surface distribution and internalization of erbB-2 proteins. Exp Cell Res 202: 274–280.
Maitra S, Kulikaukas RM, Gavilan H, Fehon RG . (2006). The tumor suppressors Merlin and expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 16: 702–709.
McClatchey AI, Giovannini M . (2005). Membrane organization and tumorigenesis—the NF2 tumor suppressor, merlin. Genes Dev 19: 2265–2277.
Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J et al. (1997). Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Dev Biol 185: 215–228.
Monje PV, Bunge M, Wood PM . (2006). Cyclic AMP synergistically enhances neuregulin-dependent ERK and Akt activation and cell cycle progression in Schwann cells. Glia 3: 649–659.
Okada T, You L, Giancotti FG . (2007). Shedding light on Merlin's wizardry. Trends Cell Biol 17: 222–229.
Osherov N, Gazit A, Gilon C, Levitzki A . (1993). Selective inhibition of the epidermal growth factor and HER2/neu receptors by tryphostins. J Biol Chem 268: 11134–11142.
Rong R, Surace EI, Haipek CA, Gutmann DH, Ye K . (2004). Serine 518 phosphorylation modulates merlin intramolecular association and binding to critical effectors important for NF2 growth suppression. Oncogene 23: 8447–8454.
Rosenbaum C, Karyala S, Marchionni MA, Kim HA, Krasnoselsky AL, Happel B et al. (1997). Schwann cells express NDF and SMDF/n-ARIA mRNAs, secrete neuregulin, and show constitutive activation of erbB3 receptors: evidence for a neuregulin autocrine loop. Exp Neurol 148: 604–615.
Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C et al. (1993). Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363: 515–521.
Scoles DR, Qin Y, Nguyen V, Gutmann DH, Pulst SM . (2005). HRS inhibits EGF receptor signaling in the RT4 rat schwannoma cell line. Biochem Biophys Res Commun 335: 385–392.
Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I et al. (2001). The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 1: 63–72.
Taylor AR, Geden SE, Fernandez-Valle C . (2003). Formation of a beta 1 integrin signaling complex in Schwann cells is independent of rho. Glia 41: 94–104.
Thaxton C, Lopera J, Bott M, Baldwin ME, Kalidas P, Fernandez-Valle C . (2007). Phosphorylation of the NF2 tumor suppressor in Schwann cells is mediated by Cdc42-Pak and requires paxillin binding. Mol Cell Neurosci 34: 231–242.
Trofatter JA, MacCollin MM, Rutter JL, Murrell JR, Duyao MP, Parry DM et al. (1993). A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72: 791–800.
Turner CE . (2000). Paxillin interactions. J Cell Sci 113: 4139–4140.
Utermark T, Kaempchen K, Hanemann CO . (2003). Pathological adhesion of primary human schwannoma cells is dependent on altered expression of integrins. Brain Pathol 13: 352–363.
Xiao GH, Beeser A, Chernoff J, Testa JR . (2002). p21-activated kinase links Rac/Cdc42 signaling to merlin. J Biol Chem 277: 883–886.
Yang D, Beirman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM et al. (2005). Coordinate control of axon defasiculation and myelination by laminin-2 and laminin-8. J Cell Biol 168: 655–666.
Acknowledgements
We thank M Marchionni and G Bokoch for graciously providing NRG1β and Pak constructs, respectively. This work was supported by a grant from DOD (award DAMD17-03-1-0211) to CFV.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thaxton, C., Lopera, J., Bott, M. et al. Neuregulin and laminin stimulate phosphorylation of the NF2 tumor suppressor in Schwann cells by distinct protein kinase A and p21-activated kinase-dependent pathways. Oncogene 27, 2705–2715 (2008). https://doi.org/10.1038/sj.onc.1210923
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1210923
Keywords
This article is cited by
-
Vestibular Schwannoma: What We Know and Where We are Heading
Head and Neck Pathology (2020)
-
Role of Merlin/NF2 inactivation in tumor biology
Oncogene (2016)
-
A neuronal function of the tumor suppressor protein merlin
Acta Neuropathologica Communications (2014)
-
LIM domain kinases as potential therapeutic targets for neurofibromatosis type 2
Oncogene (2014)
-
Rac1 is required for Prkar1a-mediated Nf2 suppression in Schwann cell tumors
Oncogene (2013)