Abstract
Pharmacological modulation of synaptic efficacy is a prominent target in the identification of promnesic compounds. Here, we report that pretraining administration of the serotonin 5-HT4 receptors (5-HT4Rs) partial agonist SL65.0155 enhances simultaneous olfactory discrimination performance and potentiates learning-induced dendritic spine growth in the mouse hippocampus. SL65.0155 does not affect spine density in the pseudo-trained mice and, by itself, does not promote spine growth. Injecting the 5-HT4 antagonist RS39604 prior to SL65.0155 prevents both the increase in performance and the additional formation of spines, thus confirming the 5-HT4Rs specificity of the observed effects. These findings provide evidence that 5-HT4Rs stimulation selectively increases experience-dependent structural plasticity in learning-activated hippocampal circuits.
Similar content being viewed by others
INTRODUCTION
Cellular mechanisms of learning and memory include the formation of new synapses and/or the remodeling of existing ones. While changes in strength and efficacy of existing synapses (ie long-term potentiation (LTP)-induced mechanisms) are widely accepted as a physiological mechanism showing parallels with learning and memory (see Morris et al, 2003 for a review), there is evidence that another form of plasticity involving rearrangements of dendritic material participates in the increase of synaptic transmission (Muller et al, 2000). Pharmacological modulation of synaptic efficacy is therefore a prominent target in the identification of drugs capable to improve or repair memory.
5-HT4 receptors (5-HT4Rs) are G-protein-coupled serotonin receptors highly expressed in various regions of the mammalian brain (Bockaert et al, 2004). Stimulation of 5-HT4Rs increases cAMP production, stimulates protein kinase A (PKA) activity and augments neuronal excitability in central nervous system neurons (Dumuis et al, 1988; Torres et al, 1996). This effect is the result of the inhibition of K+ channels, including Ca2+-activated K+ channels, that reduces the membrane after-hyperpolarization period and broadens the action potential. Stimulation of 5-HT4Rs also activates extracellular signal-regulated kinase (ERK) pathways in a Gs/cAMP/PKA-independent manner (Barthet et al, 2007) thereby affecting the presynaptic regulation of cellular plasticity (Kushner et al, 2005). Such 5-HT4Rs-mediated signaling events are likely involved in the well-established promnesic role of 5-HT4 agonists (Moser et al, 2002; Micale et al, 2007). Consistent with this view, the 5-HT4 agonist prucalopride and the potent partial agonist SL65.0155 increase LTP at hippocampal CA3–CA1 synapses (Spencer et al, 2004). Similarly, the partial agonist RS67333 prolongs LTP, prevents depotentiation of dentate gyrus neurons following perforant pathway stimulation (Kulla and Manahan-Vaughan, 2002; Marchetti et al, 2004), and shifts the frequency-dependence for hippocampal long-term depression in LTP induction (Kemp and Manahan-Vaughan, 2005). We recently reported that mice trained in a simultaneous olfactory discrimination task showed an increase in spine density on pyramidal neurons laying in the CA1 region of the hippocampus (Restivo et al, 2006). Based on the efficacy of 5-HT4 agonists as cognitive and synaptic plasticity enhancers, here we examine the effectiveness of the partial agonist SL65.0155 in improving olfactory discrimination and increasing learning-induced hippocampal spine growth.
MATERIALS AND METHODS
Animals
C57BL/6J (B6, N=59) male mice purchased from Charles River IFFA-CREDO (L'Arbresle, France) were individually housed in a temperature-controlled room (24°C) with a light : dark 12 : 12 cycle. They were given food and water ad libitum and, at the beginning of the experiments, their weights ranged from 20 to 22 g. The experiments were carried out in accordance with the guidelines laid down by the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Apparatus
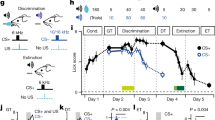
The olfactory tubing maze (OTM) (Roman et al, 2002) consisted of four discrimination chambers connected by curved transparent plastic tubes of 5 cm in diameter, with one tube having an opening on the top for placing the mouse in the apparatus (Figure 1a and Movie in Supplementary Material). The chambers were cubic enclosures (10 cm in side). Two olfactory cues were ejected simultaneously from air ports situated at extremity of two linear tubes (5 cm in diameter and 20 cm in length) branching out perpendicularly from opposite sides of each chamber. A water port in the shape of a well and a buzzer were located at the end of the straight tubes close to the air ports (Figure 1a). Entry to, and exit from, the discrimination chamber were achieved by the opening of automated doors. The solenoid and needle valves, the buzzer, and the automatic doors were controlled by a microcomputer running LabVIEW (National Instruments France). The same LabVIEW software recorded the behavioral data.
SL65.0155 enhances olfactory simultaneous discrimination performance. (a) Overview of the OTM and detail of one tube extremity hosting air and water ports for odors and reward delivery. (b) The percentage of correct choices increased across training sessions in saline-injected mice (N=7) while performance remained at a random level in pseudo-trained mice injected with saline (N=6) or SL65.0155 (N=6). Trained mice injected with SL65.0155 (N=7) displayed a higher percentage of correct choices compared to their saline-injected counterpart during the fourth and sixth training sessions. *p<0.05. (c) The intertrial intervals (ITIs) decreased similarly across sessions in all groups indicating no group difference in the learning of the procedural aspects of the OTM task. Data are expressed as mean±SEM.
Pretraining and Water Deprivation Schedule
Mice were water deprived (water available 5 min per day in the home cage) 5 days before the beginning of the experiment and maintained under this regimen during the entire experiment. Mice were then subjected to three pretraining sessions (one per day) during which they were allowed to explore the maze for 15 min with all the water ports filled with 0.1 ml of water. Training started on the fourth day. During pretraining and training, water was available immediately after the mice were returned to their home cage.
Training
Mice were placed in the maze using the dedicated curved tube. The crossing of a first photoelectric cell beam in proximity to the chamber opened the automated door. Inside the chamber, the crossing of a second photoelectric cell beam activated the timer for response latency and the two odors ejection. One odor (Allyl heptanoate; 2.5‰ concentration; positive odor—Sigma-Aldrich, France) was associated with the positive reward (0.03 ml of water) while the other one (citral dimethyl acetal, mixture of cis and trans; 5‰ concentration; negative odor—Sigma-Aldrich, France) was associated with a 3-s buzzer sound. After mice chose one odor and entered the corresponding tube, they crossed a third photoelectric cell beam activating either the buzzer or the water delivery and simultaneously opened and closed the exit and entrance doors. A minimum delay of 15 s was set between the end and the beginning of the successive trial. A noncorrection procedure was used as no water was delivered in any tube after an error was made. The position of the odors in the left and right tubes was distributed in a pseudo-random fashion within and between training sessions to prevent mice from developing a motor strategy. Mice were trained for 6 days with one daily session. A session consisted of 20 discrimination trials. Two dependent variables were recorded: (1) the percentage of correct responses (2) the intertrial intervals (ITIs), ie the time elapsing between the response to an odor in one testing chamber and the response to an odor in the next chamber.
Drugs
The selective 5-HT4 partial agonist SL65.0155. (5-(8-amino-7-chloro-2,3-dihydro-1,4-benzodioxin-5-yl)-3-(1-(2-phenylethyl)-4-piperidinyl)-1,3,4-oxadiazol-2(3H)-one-monohydrochloride) was obtained from Sanofi-Aventis (Paris, France) and the 5-HT4 antagonist RS39604 (1-(4-amino-5-chloro-2-(3,5-dimethoxyphenyl)methyloxy)-3-(1-(2-methylsulphonylamino)ethyl) piperidin-4-yl)propan-1-one) was obtained from Tocris (Cookson Ltd., Bristol, UK). Both compounds were dissolved in sterile saline (0.9% NaCl). SL65.0155 was injected i.p. in the appropriate mice groups 30 min prior to the last four training sessions. Mice were therefore drug-free during sessions 1 and 2 to minimize the effect of the compound on novelty-induced emotional reactions (Marchetti et al, 2000). The 0.01 mg/kg dose was chosen on the basis of Moser et al (2002) data as that producing the maximal enhancement of learning performance in rats and mice of different strains. RS39604 (0.5 mg/kg) was injected 15 min prior to SL65.0155, with the second injection being administered 30 min prior to the training sessions.
Experimental Groups
In experiment 1, B6 mice (N=26) were assigned to the ‘training’ (N=14) or the ‘pseudo-training’ (N=12) condition. In each condition, half mice were injected with SL65.0155 and the other half with saline. In the ‘training’ condition, the positive odor was associated with the reward on 100% of the trials. There was no positive odor in the ‘pseudo-training’ condition as each odor was randomly associated with the reward on 50% of the trials. The brains of trained and pseudo-trained mice injected with SL65.0155 or saline, and of a control cage group injected with SL65.0155 (N=4), saline (N=4), or left undisturbed (N=4) were processed for Golgi–Cox impregnation 24 h after the last OTM session. In these mice groups, spine density was estimated on pyramidal neurons laying in the CA1 hippocampal subfield and in the primary visual cortex area. In experiment 2, B6 mice receiving RS39604+saline (N=7), SL65.0155+saline (N=7), RS39604+SL65.0155 (N=8), or two saline injections (N=7) were trained in the OTM. After the completion of the last training session, the brains (RS39604+saline, N=6, SL65.0155+saline, N=6, RS39604+SL65.0155, N=6, or two saline injections, N=4) were processed for Golgi–Cox impregnation.
Golgi–Cox Impregnation of Brain Tissue
A total of 24 h after the last training or pseudo-training session, mice from the above-described groups were anesthetized with chloral hydrate (400 mg/kg) and perfused intracardially with 0.9% saline. The brains were impregnated using a Golgi–Cox solution (Glaser and Van der Loos, 1981), stored at room temperature for 6 days, immersed in a sucrose solution (30%) for 2 days, and sectioned coronally (100 μm) with a vibratome. Sections were mounted on gelatinized slides, stained as described (Gibb and Kolb, 1998), and covered with permount.
Spine Density
Measurements were performed along dendrites of pyramidal cells laying in the CA1 region of the hippocampus and in the layer V of the primary visual cortex (area V1, Paxinos and Franklin, 2001). Three CA1 and three V1 neurons within each hemisphere were selected. Since no interhemispheric difference was detected, the data were pooled so that six neurons per brain region were considered in each subject. These raw data were subsequently averaged for an animal mean. Spines were counted on fully impregnated neurons under a high magnification (× 63/0.75 NA). Measurements were performed on apical and oblique dendrites laying in the stratum radiatum and on secondary and tertiary branches of basal dendrites laying in the stratum oriens of CA1. In the V1 region secondary and tertiary branches of basal and apical dendrites were sampled from pyramidal neurons with the soma laying in the fifth layer. On each neuron and for each dendrite category, five 20 μm dendrite segments laying on the focal plane were randomly sampled. Segments were sampled 50 μm away from soma in order to exclude the spine-depleted zone which arise from the cell body. The average spine density (number of spines per 10 μm dendritic length) was estimated focusing in and out with the fine adjustment of the microscope (Leica DMLB). All morphological measurements were performed by an experimenter blind to the experimental condition of the animals.
Statistics
All data sets fitted to a normal distribution (Kolmogorov–Smirnov test, d<0.334, for all analyses). In experiment 1, the percentage of correct responses, the values of ITIs and the percentage of weight loss were compared by means of a three-way ANOVA with ‘training condition’ and ‘treatment’ as between-group factors and ‘sessions’ as within-group factor. In experiment 2, the same variables were compared by means of a two-way ANOVA with ‘treatment’ and ‘sessions’. Differences in spine density estimated by means of one-way ANOVAs with ‘experimental condition’ as between-group factor. Spine density values (six neurons per brain region) were averaged for each mouse and compared among groups. Post hoc pair-wise comparisons were carried out where necessary using the Newmann–Keuls' test. p-level was set at 0.05 for all statistical tests.
RESULTS
Experiment 1
Body weight
Water deprivation produced the same decrease in body weight in all the mice groups (significant main effect of sessions: F(8, 176)=318.25; p<0.001—Supplementary Figure S1).
Effects of 0.01 mg/kg SL65.0155 on simultaneous olfactory discrimination performance
Simultaneous olfactory discrimination in the OTM is an example of flexible memory expression requiring computation and storage of multiple stimulus-outcome representations. These operations match the notion of declarative memory as defined by Eichenbaum et al (1988) and require an intact hippocampus to be successfully implemented (Myers and Gluck, 1996). To assess the effect of 5-HT4R agonists on declarative cognitive processing, we compared the percentage of correct discrimination responses in trained and pseudo-trained mice injected with SL65.0155 or saline in the OTM. The data are shown in Figure 1b. Results showed a significant main effect of training condition (F(1, 22)=129.89; p<0.001), Treatment (F(1, 22)=9.97; p<0.01), sessions (F(5, 110)=12.53; p<0.001), and a significant training condition × treatment × sessions (F(5, 110)=2.38; p<0.05) interaction. This indicated that mice exposed to a 100% rewarded odor showed increased discrimination performance across sessions relative to pseudo-trained mice exposed to two randomly rewarded odors (trained mice injected with SL65.0155 or saline vs pseudo-trained mice injected with SL65.0155 or saline, p<0.05 for all comparisons). However, among the trained mice, those injected with SL65.0155 performed significantly better during the fourth and sixth training sessions (p<0.05 for both comparisons) than saline-injected mice.
Effects of 0.01 mg/kg SL65.0155 on the acquisition of motor procedures
In parallel to the declarative component of the simultaneous olfactory discrimination paradigm, the learning of the OTM task requires the mastering of motor habits that need to be gradually automated during the course of training. Motor habits are a form of procedural learning independent of the hippocampus (White and McDonald, 2002). In the OTM, the time elapsing between two consecutive discrimination trials (ITIs) estimates how motor habits develop. We examined whether this variable varied according to the training or the treatment condition across sessions. Results revealed only a significant main effect of the session factor for ITI (F(5, 110)=13.00, p<0.001) indicating that trained and pseudo-trained mice similarly learnt OTM motor procedures and that SL65.0155 did not affect this form of learning (Figure 1c).
Effects of 0.01 mg/kg SL65.0155 on learning-induced spine growth on CA1 neurons dendrites
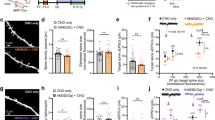
Learning-induced structural changes on CA1 pyramidal neurons have been shown in a variety of hippocampal-dependent tasks including trace fear conditioning (Leuner et al, 2003), spatial learning (Moser et al, 1997), olfactory-rule learning (Knafo et al, 2005), and simultaneous olfactory discrimination (Restivo et al, 2006). Based on the efficacy of SL65.0155 in increasing OTM performance, we examined whether the promnesic effect of this compound was accompanied by a potentiation of learning-induced hippocampal spine growth. Group differences in the cumulative frequency of spine density at each dendritic locus and representative examples of spine density on oblique dendrites are shown in Figures 2a–b. Data of pseudo-trained mice injected with SL65.0155 or saline were pooled together as no variation in spine density was found between these two groups (apical: t(6)=0.32; oblique: t(6)=−0.31; basal: t(6)=−0.26, NS for all comparisons). These data were referred as pseudo-trained group data. In the same fashion, data from control cage mice treated with SL65.0155 or saline were pooled together as the 5-HT4 agonist was not found to affect spine growth (apical: F(2, 9)=0.15; oblique: F(2, 9)=0.4; basal: F(2, 9)=0.17, NS for all comparisons—Supplementary Figure S2). These data were referred as control cage data. One-way ANOVAs revealed consistent group differences in spine density on all dendrites categories (apical: F(3, 24)=22.47 p<0.0001; oblique: F(3, 24)=44.51; p<0.0001; basal: F(3, 24)=22.85; p<0.001). Pair-wise comparisons then showed that control cage and pseudo-trained mice did not differ in the number of spines for any dendrite category except for a small difference detected on oblique dendrites (oblique dendrites, p<0.05). As expected (Restivo et al, 2006), these two nontrained groups exhibited less spines than saline-injected trained mice in all dendrite compartments (p<0.01 for all comparisons); however, trained mice injected with SL65.0155 exhibited a further increase in spines relative to trained mice injected with saline (apical dendrites, p<0.01; oblique dendrites, p<0.05; basal dendrites, p<0.05).
SL65.0155 potentiates learning-induced spine growth. (a) Cumulative frequency distribution of spine density measured along apical, oblique, and basal dendrites of CA1 pyramidal neurons. Shifting of the curves to the right indicates an increase in spine density on the majority of sampled neurons (SL65.0155 (▴): N=36; saline (•): N=36; pseudo (○): N=48; control (C): N=48). Insets depict the average spine density (spines per 10 μm) per group (SL65.0155: N=6; saline: N=6; pseudo: N=8; control: N=8). Saline-injected mice trained in the OTM showed a significant increase in spine density along apical, oblique, and basal dendrites compared to pseudo-trained and control mice. Pseudo-trained mice in the OTM increased spine density on oblique dendrites. SL65.0155 promoted a further increase of spines on all dendrite categories of trained mice. *p<0.05; #p<0.01. (b) Photomicrographs of representative Golgi–Cox impregnated oblique dendrites on CA1 pyramidal neurons of the experimental groups. Scale bar=1 μm.
Effects of 0.01 mg/kg SL65.0155 on learning-induced spine growth on primary visual cortex neurons dendrites
To control that the increase in spine density found in the trained mice was specific to the hippocampus, we counted dendritic spines on V1 pyramidal neurons in the same mice groups. One-way ANOVAs revealed no group difference for any dendrite category in this region (apical dendrites, F(3, 20)=0.50; NS; oblique dendrites F(3, 20)=0.13; NS; basal dendrites F(3, 20)=0.72; NS, Supplementary Figure S3).
Experiment 2
Body weight
In all groups, water deprivation produced the same decrease in body weight (significant main effect of sessions: F(8, 200)=373.51; p<0.001—Supplementary Figure S4).
Effects of 0.5 mg/kg rs39604 on 0.01 mg/kg SL65.0155-dependent increase in olfactory discrimination performance and learning-induced spine growth on CA1 dendrites
To determine whether the behavioral and neuronal changes observed in trained mice receiving SL65.0155 injections were specifically due to the activation of 5-HT4Rs, we performed additional experiments in which the selective 5-HT4R antagonist RS39604 was injected 15 min prior to SL65.0155 in mice subsequently subjected to OTM training. These mice were compared to mice receiving a saline injection 15 min prior to SL65.0155, to RS39604, or to a second saline injection. The discrimination scores and the ITIs recorded in these four groups are shown in Figures 3a–b. For the discrimination scores, no effect of the treatment (F(3, 25)=2.37, NS) but a significant effect of sessions (F(5, 125)=25.59, p<0.001) and of the treatment × sessions interaction (F(15, 125)=2.09; p<0.05) was found. Post hoc comparisons revealed similar discrimination scores in mice receiving RS39604 plus SL65.0155, saline plus R639604, or the double injection of saline while mice receiving saline plus SL65.0155 performed still better during the fourth (p<0.05) and the sixth (p<0.05) training sessions. For the ITIs, there was only an effect of sessions (F(15, 125)=17.55; p<0.001) indicating that all mice similarly learned OTM motor procedures. We then compared spine density on CA1 dendrites in these four mice groups. The data are shown in Figure 4. One-way ANOVAs revealed significant group differences for each dendrite category (apical: F(3, 20)=8.46, p<0.001; oblique: F(3, 20)=3.97; p<0.05; basal: F(3, 20)=4.97; p<0.01). Post hoc comparisons then indicated that mice receiving saline plus SL65.0155 exhibited more spines on apical and oblique dendrites compared to the three other groups among which no difference was found. In the basal dendrite compartment, however, these mice still showed more spines than those injected with saline plus RS39604 (p<0.05) and with RS39604 plus SL65.0155 (p<0.01), but not than those receiving the double saline injection.
Blocking 5-HT4Rs by injecting RS39604 prior to SL65.0155 prevented the increase in performance. (a) Mice injected with saline+SL65.0155 exhibited a higher percentage of correct choices compared to all other experimental groups during the fourth and sixth sessions. (b) The intertrial intervals (ITIs) decreased similarly across training sessions in all groups. Data are expressed as mean±SEM. *p<0.05; #p<0.01.
Blocking 5-HT4Rs by injecting RS39604 prior to SL65.0155 prevented the potentiation of learning-induced spine growth. Cumulative frequency distribution of spine density measured along apical, oblique, and basal dendrites of CA1 pyramidal neurons. Shifting of the curves to the right indicates an increase of spine density on the majority of sampled neurons (saline+SL65.0155 (▴): N=36; saline+saline (○): N=36; saline+RS39604 (⋄): N=36; RS39604+SL65.0155 (⋄): N=36). Insets depict the average spine density (spines per 10 μm) per group (N=6 mice, for all groups). (a) Saline+SL65.0155 group showed a significant increase in spine density along apical and oblique dendrites relative to all experimental groups. *p<0.05. (b) Photomicrographs of representative Golgi–Cox impregnated oblique dendrites on CA1 pyramidal neurons of the experimental groups. Scale bar=1 μm.
DISCUSSION
The main finding of the present experiments is that pretraining injections of the 5-HT4 partial agonist SL65.0155 caused an enhancement of simultaneous discrimination scores and a potentiation of learning-induced spine growth on CA1 hippocampal neurons in C57BL/6 mice. Saline-injected mice showed about 70% of correct discrimination choices at the end of training and exhibited a 10% increase of dendritic spines relative to their pseudo-trained counterpart. This increase is therefore similar to that reported in noninjected B6 mice subjected to the same olfactory discrimination task (Restivo et al, 2006), and appears in the same range as that found in rats trained in a spatial (12%, Moser et al, 1997) or in an olfactory-rule learning task (10%, Knafo et al, 2005). However, mice injected with SL65.0155 showed higher discrimination scores (85%) and exhibited a further increase of spines of about 6% relative to mice injected with saline. Remarkably, administration of SL65.0155 in trained or pseudo-trained mice did not affect the procedural component of the learning task estimated through the progressive reduction of ITIs. In the same mice, the compound was not found modifying spine density on pyramidal neurons laying in the primary visual cortex area, a region unrelated to the memory pathways. Thus, considering those changes in hippocampal synaptic connectivity are required for the stabilization of neuronal circuits underlying newly formed memories (Eichenbaum, 2004), the present data demonstrate the possibility of boosting rewiring in learning-activated circuits through simulation of 5-HT4Rs. Because SL65.0155 did not enhance spine density in the pseudo-trained mice and, by itself, did not promote spine growth in the control cage mice, it can be assumed that it selectively increased hippocampal structural plasticity in experimental animals required to process meaningful information. The 5-HT4Rs specificity of this effect is confirmed by the fact that injecting the 5-HT4 antagonist RS39604 prior to SL65.0155 prevented the increase in performance and the potentiation of learning-induced spines, with mice receiving the two compounds or the double injection of saline showing comparable levels of performance and of dendritic spines. The lack of effect of the 5-HT4 antagonist on discrimination performance is consistent with previous studies showing that RS39604 did not affect the performance of rats in a place recognition task (Orsetti et al, 2003) nor interfered with basal synaptic transmission, LTP, or depotentiation in rat hippocampal slices (Kulla and Manahan-Vaughan, 2002). These findings support the view that the increment in OTM performance does not crucially depend on the endogenous activity of serotonin but, rather, on 5-HT4Rs activation enhancing excitability in central nervous system neurons (Dumuis et al, 1988; Torres et al, 1996).
Interestingly, we observed that spine growth was increased in all dendrite compartments that do not serve the same function. Apical and oblique dendrites of CA1 neurons receive afferents from the Schaffer's collaterals laying in the same hemisphere and therefore connect CA3 to CA1 regions within the left or the right hippocampus. Basal dendrites also ensure CA3 to CA1 intrahemispheric connections but receive a stronger contralateral input making them extensively involved in intrahemispheric neurotransmission (Swanson et al, 1978). Another characteristic of basal dendrites is their major propensity to undergo plastic changes (Kaibara and Leung, 1993). In particular, these dendrites exhibit a greater magnitude of LTP (Toth and Freund, 1992) that may explain why learning-induced spine growth has been until now prevalently observed in the basal compartment (Leuner et al, 2003; Knafo et al, 2005). Thus, although task- or species-related factors may contribute in the present spine growth pattern, the observation that spine density was increased at all dendritic loci in trained mice injected with SL65.0155 suggests that activation of 5-HT4Rs globally enhances connectivity and excitatory neurotransmission in learning-activated hippocampal circuits. Additional support for a role of 5-HT4Rs in the remodeling of experience-induced synaptic contacts comes from data showing that induction of late facilitation of synaptic transmission (L-LTP) in amygdala slices by local applications of the 5-HT4 agonist RS67333 requires both protein synthesis and cytoskeletal rearrangements (Huang and Kandel, 2007). Thus, 5-HT4Rs-mediated L-LTP elicits neuronal rewiring in the amygdala presumably favoring the storage of emotional memories in amygdalar circuits.
We mentioned above that this effect may be due to the 5-HT4R modulation of PKA signaling considering that (i) 5-HT4Rs agonists activate cAMP production and PKA activity (Bockaert et al, 2004; Dumuis et al, 1988), and (ii) a direct relationship exists between PKA-related calcium permeability of NMDA receptors and experience-dependent synaptic remodeling (Skeberdis et al, 2006). Indeed, the preferential location of 5-HT4Rs on the somata of hippocampal glutamatergic CA1 cells (Bickmeyer et al, 2002) is likely to play a crucial role in this process. Stimulation of 5-HT4Rs also produces a potent but transient activation of the ERK pathway (Barthet et al, 2007). Knowing that BDNF/TrkB signaling increases spine density in hippocampal CA1 pyramidal neurons (Tyler and Pozzo Miller, 2001, 2003), but that the effects of BDNF on dendritic spine growth requires activation of ERK (MAPK) signaling, it could be that, in addition to classical cAMP/PKA pathways, 5-HT4Rs may use ERK pathways to control hippocampal plastic changes and memory.
Collectively, our findings provide evidence that a systemically injected pharmacological promnesic compound enhances simultaneous olfactory discrimination performance and increases a core mechanism of hippocampal plasticity, namely, learning-induced spine growth. While confirming the therapeutic potential of 5HT4 agonists in cognitive pathologies associated with temporal lobe dysfunction, these observations indicate the possibility of estimating and comparing the efficacy of memory enhancers through their capacity of stimulating structural plasticity in learning-activated neural circuits.
References
Barthet G, Framery B, Gaven F, Pellissier L, Reiter E, Claeysen S et al. (2007). 5-HT4 receptor activation of the ERK pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell 6: 1979–1991.
Bickmeyer U, Heine M, Manzke T, Richter DW (2002). Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur J Neurosci 16: 209–218.
Bockaert J, Claeysen S, Compan V, Dumuis A (2004). 5-HT4 receptors. Curr Drug Targets CNS Neurol Disord 3: 39–51.
Dumuis A, Bouhelal R, Sebben M, Bockaert J (1988). A 5-HT receptor in the central nervous system, positively coupled with adenylate cyclase, is antagonized by ICS 205 930. Eur J Pharmacol 146: 187–188.
Eichenbaum H (2004). Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120.
Eichenbaum H, Fagan A, Mathews P, Cohen NJ (1988). Hippocampal system dysfunction and odor discrimination learning in rats: impairment or facilitation depending on representational demands. Behav Neurosci 102: 331–339.
Gibb R, Kolb B (1998). A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods 79: 1–4.
Glaser EM, Van der Loos H (1981). Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high clarity Golgi-Nissl stain. J Neurosci Methods 4: 117–125.
Huang YY, Kandel ER (2007). 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci 27: 3111–3119.
Kaibara T, Leung LS (1993). Basal vs apical dendritic long-term potentiation of commissural afferents to hippocampal CA1: a current-source density study. J Neurosci 13: 2391–2404.
Kemp A, Manahan-Vaughan D (2005). The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region in vivo. Cereb Cortex 15: 1037–1043.
Knafo S, Libersat F, Barkai E (2005). Dynamics of learning-induced spine redistribution along dendrites of pyramidal neurons in rats. Eur J Neurosci 21: 927–935.
Kulla A, Manahan-Vaughan D (2002). Modulation by serotonin 5-HT(4) receptors of long-term potentiation and depotentiation in the dentate gyrus of freely moving rats. Cereb Cortex 12: 150–162.
Kushner SA, Elgersma Y, Murphy GG, Jaarsma D, van Woerden GM, Hojjati MR et al. (2005). Modulation of presynaptic plasticity and learning by the H-ras/extracellular signal-regulated kinase/synapsin I signaling pathway. J Neurosci 25: 9721–9734.
Leuner B, Falduto J, Shors TJ (2003). Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci 23: 659–665.
Marchetti E, Chaillan FA, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS (2004). Modulation of memory processes and cellular excitability in the dentate gyrus of freely moving rats by a 5-HT4 receptors partial agonist, and an antagonist. Neuropharmacology 47: 1021–1035.
Marchetti E, Dumuis A, Bockaert J, Soumireu-Mourat B, Roman FS (2000). Differential modulation of the 5-HT(4) receptor agonists and antagonist on rat learning and memory. Neuropharmacology 39: 2017–2027.
Micale V, Marco Leggio G, Mazzola C, Drago F (2007). Cognitive effects of SL65.0155, a serotonin 5-HT(4) receptor partial agonist, in animal models of amnesia. Brain Res 1121: 207–215.
Morris RG, Moser EI, Riedel G, Martin SJ, Sandin J, Day M et al. (2003). Elements of a neurobiological theory of the hippocampus: the role of activity-dependent synaptic plasticity in memory. Philos Trans R Soc Lond B Biol Sci 358: 773–786.
Moser MB, Trommald M, Egeland T, Andersen P (1997). Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. J Comp Neurol 380: 373–381.
Moser PC, Bergis OE, Jegham S, Lochead A, Duconseille E, Terranova JP et al. (2002). SL65.0155, a novel 5-hydroxytryptamine(4) receptor partial agonist with potent cognition-enhancing properties. J Pharmacol Exp Ther 302: 731–741.
Muller D, Toni N, Buchs PA (2000). Spine changes associated with long-term potentiation. Hippocampus 10: 596–604.
Myers CE, Gluck MA (1996). Cortico-hippocampal representations in simultaneous odor discrimination: a computational interpretation of Eichenbaum, Mathews, and Cohen (1989). Behav Neurosci 110: 685–706.
Orsetti M, Dellarole A, Ferri S, Ghi P (2003). Acquisition, retention, and recall of memory after injection of RS67333, a 5-HT(4) receptor agonist, into the nucleus basalis magnocellularis of the rat. Learn Mem 10: 420–426.
Paxinos G, Franklin KBJ (2001). The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego.
Restivo L, Roman FS, Ammassari-Teule M, Marchetti E (2006). Simultaneous olfactory discrimination elicits a strain-specific increase in dendritic spines in the hippocampus of inbred mice. Hippocampus 16: 472–479.
Roman FS, Marchetti E, Bouquerel A, Soumireu-Mourat B (2002). The olfactory tubing maze: a new apparatus for studying learning and memory processes in mice. J Neurosci Methods 117: 173–181.
Skeberdis VA, Chevaleyre V, Lau CG, Goldberg JH, Pettit DL, Suadicani SO et al. (2006). Protein kinase A regulates calcium permeability of NMDA receptors. Nat Neurosci 9: 501–510.
Spencer JP, Brown JT, Richardson JC, Medhurst AD, Sehmi SS, Calver AR et al. (2004). Modulation of hippocampal excitability by 5-HT4 receptor agonists persists in a transgenic model of Alzheimer's disease. Neuroscience 129: 49–54.
Swanson LW, Wyss JM, Cowan WM (1978). An autoradiographic study of the organization of intrahippocampal association pathways in the rat. J Comp Neurol 181: 681–715.
Torres GE, Arfken CL, Andrade R (1996). 5-Hydroxytryptamine4 receptors reduce after hyperpolarization in hippocampus by inhibiting calcium-induced calcium release. Mol Pharmacol 50: 1316–1322.
Toth K, Freund TF (1992). Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience 49: 793–805.
Tyler WJ, Pozzo-Miller L (2003). Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones. J Physiol 553: 497–509.
Tyler WJ, Pozzo-Miller LD (2001). BDNF enhances quantal neurotransmitter release and increases the number of docked vesicles at the active zones of hippocampal excitatory synapses. J Neurosci 21: 4249–4258.
White NM, McDonald RJ (2002). Multiple parallel memory systems in the brain of the rat. Neurobiol Learn Mem 77: 125–184.
Acknowledgements
The authors thank the three anonymous reviewers for their helpful criticism and comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosure/Conflict of Interest
The authors declare that, except for income from their primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Rights and permissions
About this article
Cite this article
Restivo, L., Roman, F., Dumuis, A. et al. The Promnesic Effect of G-protein-Coupled 5-HT4 Receptors Activation Is Mediated by a Potentiation of Learning-Induced Spine Growth in the Mouse Hippocampus. Neuropsychopharmacol 33, 2427–2434 (2008). https://doi.org/10.1038/sj.npp.1301644
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301644
Keywords
This article is cited by
-
The role of serotonin neurotransmission in rapid antidepressant actions
Psychopharmacology (2022)
-
Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in a hippocampal-dependent memory task
Translational Psychiatry (2021)
-
Serotonin 5-HT4 receptor boosts functional maturation of dendritic spines via RhoA-dependent control of F-actin
Communications Biology (2020)