Abstract
As with other drugs of abuse, heroin use is characterized by a high incidence of relapse following detoxification that can be triggered by exposure to conditioned stimuli previously associated with drug availability. Recent findings suggest that cannabinoid CB1 receptors modulate the motivational properties of heroin-conditioned stimuli that induce relapse behavior. However, the neural substrates through which CB1 receptors modulate cue-induced heroin seeking have not been elucidated. In this study, we evaluated alterations in cue-induced reinstatement of heroin-seeking behavior produced by infusions of the CB1 receptor antagonist SR 141716A (0, 0.3 and 3 μg per side) delivered into the prefrontal cortex (PFC), nucleus accumbens (NAC), and basolateral amygdala (BLA) of rats. Results show that following extinction of operant behavior the presentation of a discriminative stimulus conditioned to heroin availability reinstated nonreinforced lever pressing to levels comparable to preextinction levels. Intra-PFC SR 141716A dose-dependently reduced cue-induced reinstatement of heroin seeking, with a significant reduction following the 3 μg per side dose. In the NAC, both SR 141716A doses induced a significant reduction in cue-induced reinstatement, with the highest dose completely blocking the effect of the cue. In contrast, intra-BLA SR 141716A did not alter cue-induced reinstatement of responding while systemic administration of this antagonist (3 mg/kg, i.p.) significantly blocked cue-induced reinstatement in all three-placement groups (BLA, PFC, and NAC). These findings provide new insights into the neural mechanisms through which CB1 receptors modulate the motivational properties of heroin-associated cues inducing relapse.
Similar content being viewed by others
INTRODUCTION
Relapse to drug-seeking and drug-taking behaviors despite long periods of abstinence is a common manifestation of drug dependence (Leshner, 1997; McLellan et al, 2000). High rates of relapse are observed for different drugs of abuse (Tims and Leukefeld, 1986) turning interest towards the prevention of this phenomenon as a treatment for addiction (DeJong, 1994; McLellan et al, 2000). Self-reports by drug-dependent humans indicate that multiple factors such as exposure to drug-associated stimuli, drug priming, and stress can precipitate drug craving and relapse (Jaffe et al, 1989; O'Brien et al, 1992; Kreek and Koob, 1998; Carter and Tiffany, 1999; Sinha et al, 1999). The study of drug-associated cues as causal factors for relapse is of major interest because drug-abstinent subjects are likely to be exposed to stimuli such as drug paraphernalia and drug-associated environments. These stimuli acquire motivational salience by predicting drug availability, and thus exposure to these drug-associated stimuli can precipitate craving and drug seeking (Tims and Leukefeld, 1986).
Recently developed animal models of drug seeking have contributed substantially to our understanding of the neurobiological mechanisms underlying drug relapse (Shaham et al, 2000; Stewart, 2000; Shalev et al, 2002; See, 2005; Weiss, 2005; Zavala et al, 2007), though their construct validity (referring to the similarities in the mechanisms through which drug relapse occurs in humans vs animal models) faces intense examination (Epstein et al, 2006). Nonetheless, substantial evidence gathered using these animal models implicates the cannabinoid system in the mediation or modulation of drug-seeking behavior. For example, systemic administration of the CB1 cannabinoid receptor agonists HU210, WIN 55 212-2, and CP 55 940 reinstate heroin-seeking behavior, while the CB1 receptor antagonist SR 141716A attenuates cue-induced and heroin-primed reinstatement of heroin-seeking behavior (De Vries et al, 2003; Fattore et al, 2003). Similar findings have been made regarding relapse behavior associated with other drugs of abuse such as nicotine (De Vries et al, 2005), alcohol (Serra et al, 2002), and cocaine (De Vries et al, 2001) suggesting that cannabinoid signaling is involved in drug-seeking behavior in general, regardless of the nature of the drug being sought.
Although these findings point to the endocannabinoid (eCB) system as a potential target for the treatment of drug relapse, further studies assessing the neural substrates through which eCBs modulate reinstatement of drug seeking are needed. In this regard, it has been proposed that the prefrontal cortex (PFC), basolateral amygdala (BLA), and nucleus accumbens (NAC), respectively, participate in the encoding and recognition of predictive stimuli, the establishment of learned associations, and the expression of goal-directed behaviors in response to motivationally salient stimuli predicting drug availability (Kalivas and Volkow, 2005). Based on these findings, and the presence of CB1 receptors in the PFC, NAC, and BLA (Herkenham et al, 1991; Tsou et al, 1998; Katona et al, 2001; McDonald and Mascagni, 2001) we hypothesize that CB1 receptors in these brain regions modulate cue-induced reinstatement of heroin-seeking behavior. To examine this possibility, we used an animal model of drug-seeking behavior (Weiss et al, 2000) to test the effect of localized infusions of the CB1 receptor antagonist SR 141716A into the PFC, NAC, and BLA on the reinstatement of drug-seeking behavior induced by presentation of a heroin-associated conditioned cue. Previous work has demonstrated that this cue-induced lever pressing behavior is remarkably resistant to extinction (Gracy et al, 2000; Ciccocioppo et al, 2001a), and therefore this model is well-suited for within-subject evaluations of the dose-dependent effects of SR 141716A. Our results suggest a role for CB1 receptors in the PFC, NAC but not BLA, in the modulation of cue-induced reinstatement of heroin-seeking behavior.
MATERIALS AND METHODS
Animals
Male Wistar rats (Charles River, Raleigh, NC) weighing 200–250 g were housed three per cage on a reversed 12 h light/dark cycle with food and water available ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Drugs and Reagents
Heroin hydrochloride was obtained from the National Institute on Drug Abuse (Washington, DC, USA) and was dissolved in a vehicle of sterile 0.9% saline. SR 141716A was generously provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Washington, DC, USA) and was dissolved in a vehicle of ethanol: emulphor: saline (1:1:18).
Surgery
Twenty-nine rats were prepared with chronic silastic jugular catheters under isoflurane anesthesia (1.5–2.0%) as described elsewhere (Caillé and Parsons, 2006). During this same surgery the animals were also stereotaxically implanted with bilateral 22-gauge, 12-mm stainless steel guide cannulae that terminated 2 mm above the brain region of interest. Separate groups of animals received bilateral guide cannulae aimed at the NAC core, medial PFC, and BLA.
These brain subregions were chosen based on the presence of CB1 receptors in these areas (Mailleux and Vanderhaeghen, 1992; Matsuda et al, 1993; Tsou et al, 1998; Egertova and Elphick, 2000) and evidence for an involvement of each region in the mediation of cue-induced reinstatement of cocaine- and heroin-seeking behavior and that inactivation of either the BLA or medial PFC abolishes cue- and contextual-induced reinstatement of cocaine and heroin-seeking behavior (Meil and See, 1997; Di Ciano and Everitt, 2001; Fuchs and See, 2002; McLaughlin and See, 2003; Fuchs et al, 2005). The stereotaxic coordinates were: NAC core (from Bregma: AP, +1.7 mm, ML, ±2.0 mm and V, −5.0 mm (from dura; n=12)); PFC (AP, +3.0 mm, ML, ±0.6 mm, and V −3.6 mm (from dura; n=8)); BLA (AP, −2.56 mm, ML, ±4.8 mm, and V –6.0 mm (from dura; n=9)). Vertical placements were used for each brain region, using a Kopf ultra-precise small animal stereotaxic apparatus (model 963; David Kopf Instruments, Tujunga California). The i.v. catheter inlet fixture and injector cannulae were secured to the skull with cranioplastic cement over skull screws implanted in each quadrant. Catheters were flushed daily with sterile heparinized saline (30 USP units/ml) and the animals were allowed a minimum of seven postoperative recovery days prior to the initiation of self-administration training.
Intravenous Heroin Self-Administration Training
The self-administration chambers consisted of operant boxes enclosed in sound attenuating, fan ventilated environmental cubicles (MED Associates Inc., St Albans, VT or BRS/LVE, Laurel, MD). Each rat learned the operant self-administration task by autoshaping in 2 h sessions conducted 5 days per week. At the start of each session two operant levers were extended into the chamber. Responding on the active lever was reinforced by the intravenous infusion of 0.1 ml of a heroin solution (0.01 mg per infusion delivered over 4 s) under a continuous reinforcement schedule (FR-1) with a 20 s timeout period following each drug infusion during which lever responses were recorded but not reinforced. Responses on the inactive lever were recorded but had no programmed consequence. The orientation of the active and inactive levers (left or right side) was counterbalanced between animals, but remained constant for the duration of each animals' testing period. The S+ environment associated with heroin self-administration consisted of a speaker generated constant white noise (70 dB) along with the illumination of a cue light (compound discriminative stimuli) above the active lever for 20 s when the lever was pressed. The initial period of 2 h daily self-administration training continued for 2 weeks, during which time all animals acquired self-administration behavior as evidenced by an average of 18±1.3 heroin infusions obtained per session during the final two training sessions.
Cue Discrimination Training
Following the initial self-administration training each group was subjected to a period of cue discrimination training designed to condition distinct discriminative stimuli with heroin availability and nonavailability. During this phase of training each animal received three 1 h self-administration sessions, each separated by 30 min. During two of these sessions responding on the active lever produced an intravenous heroin infusion (0.01 mg/ml), and these sessions were associated with the S+ environment described above (continuous white noise and illumination of a cue light above the lever when it is pressed). During the remaining daily session responses on the active lever produced an intravenous saline infusion (0.1 ml), and this session was associated with a distinct set of environmental cues (S− environment; continuous house light illumination and presentation of an intermittent tone (70 dB, 20 s duration following lever press) as the compound discriminative stimuli). In each of these sessions responses on the inactive lever were recorded but not reinforced. Discrimination training was conducted 5 days per week until the animals' behavior reached the baseline criterion of 3 consecutive days of ⩽5 lever presses on the active lever during the saline self-administration (S−) sessions.
Extinction
Once the baseline criterion for cue discrimination was met each animal was put through a series of 1 h extinction sessions that were conducted 5 days per week. Extinction sessions were initiated by the extension of both levers but without presentation of any discriminative stimuli. Responses on the previously active lever engaged the syringe pump motor for 4 s, but did not result in any drug infusion or presentation of a discriminative stimulus. Responses on the previously inactive lever were without consequence. Extinction sessions were continued until the animals reached the criterion of 3 consecutive days with ⩽−5 lever presses per session on the previously active lever. During the final week of extinction training a sham microinfusion was performed (eg microinjectors inserted through the guide cannulae but no solution administered). This was done to avoid biasing subsequent reinstatement responding with possible behavioral alterations produced by the initial microinjector insertion.
Cue-Induced Reinstatement of Drug Seeking
Reinstatement tests were conducted on eight occasions over the course of 23 days after the extinction of operant behavior. The heroin-associated (S+) and saline-associated (S−) cues were reintroduced in these 1 h tests, though operant responding did not result in any drug or saline infusions. Sessions began by the extension of the levers and the presentation of either the S+ or S− environmental cue (white noise or house light, respectively), and these stimuli remained present until retraction of the levers at the end of each session. During the session responses at the previously active lever resulted in activation of the syringe pump and a 20-s timeout (signaled by illumination of the cue light above the right lever in the S+ condition, and the intermittent tone in the S− condition). Responses at the previously active lever were measured as an index of cue-induced reinstatement of extinguished heroin seeking while responses at the inactive lever were without consequence and were recorded as a measure of behavioral specificity.
To verify the behavioral selectivity of the discriminative stimuli, all rats were tested in the presence of the S− on the first and last reinstatement testing days (days 1 and 23, respectively). The effects of intracranial SR 141716A infusion on S+-induced reinstatement of heroin seeking were evaluated in three 1 h tests each separated by 3 nontesting days (eg tests performed on reinstatement days 2, 5, and 8, respectively). Infusions were made via bilateral 33-gauge microinjectors that extended 2 mm beyond the tip of the guide cannulae. The microinjectors were constructed such that the 33-gauge injector tubing passes through and extends 2 mm beyond a length of 26-gauge tubing which is used to increase the outer diameter of the injection cannula, thereby increasing the rigidity of the injector and ensuring a tighter fit between the injection and guide cannulae. This increases the accuracy of injector placement and minimizes injector movement when inserted through the guide cannula. Each animal was held during the intracranial injections to ensure that the infusion cannulae did not rotate or move while in tissue. However, to minimize stress this handling did not involved immobilization or excessive restraint. About 1 μl per side infusions were made at a rate of 0.5 μl/min using 10 μl Hamilton syringes mounted on a Harvard Apparatus syringe pump (model 975). The infusion cannulae were left undisturbed for 1 min following completion of the infusion to allow drug diffusion prior to injector removal. Subsequently, stylets were replaced in the guide cannulae and the rats were immediately tested for cue-induced reinstatement of responding. The effects produced by vehicle, 0.3 or 3.0 g per side SR 141716A were evaluated and the dose presentation was randomized between animals. Each animal was tested once with each dose. To evaluate cue-induced heroin seeking in the absence of any pretreatment manipulation an S+ session was performed on day 14 of the reinstatement phase.
One week after the final micro-infusion test the effects of intraperitoneal SR 141716A administration on cue-induced reinstatement of heroin seeking were evaluated. Animals were injected either with vehicle or SR 141716A (3 mg/Kg, i.p.), then were placed back into their home cages for 30 min until testing. These evaluations of systemically administered SR 141716A on S+-induced heroin seeking were performed on days 17 and 20 (pretreatment order randomized between animals).
Histological Verification of Infusion Cannulae Placement
Following the final behavioral test each rat was euthanized by isoflurane overdose, the brain was removed and immediately frozen on dry ice. Subsequently the brain was mounted on a cryostat and sliced into 50 μm sections. Based on the presence of tissue damage from injector insertion and drug infusion injector placements within the regions of interest were evaluated based on the stereotaxic boundaries as described by of Paxinos and Watson (1998). Only those subjects with accurate bilateral placements were included in the final data analyses.
Statistical Analyses
One way analyses of variance (ANOVA) were used to evaluate differences in lever pressing behavior during discrimination training (S+ vs S− sessions), to compare lever pressing behavior in the presence of the S− or S+ discriminative stimuli vs responding under extinction conditions, and to evaluate the effects of either intracranial or peripheral SR 141716A pretreatment on cue-induced heroin-seeking behavior. The data used for these comparisons were the number of reinforced responses per hour exclusive of any responding during the timeout period (timeout responses were rare following initial self-administration training). Post hoc analyses were made using Fisher's PLSD. Data are expressed as the mean±SEM.
RESULTS
Intra-NAC SR 141716A Infusion Dose-Dependently Decreases Cue-Induced Heroin-Seeking Behavior
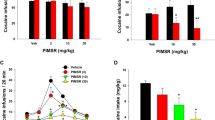
Rats in the NAC placement group (n=12) readily acquired heroin self-administration and demonstrated cue-discrimination. During the final 3 days of cue discrimination training rats in this group elicited significantly greater lever pressing on the active lever during the S+ heroin sessions than during the S− saline sessions (8.17±0.9 vs 2.2±0.7 lever presses per session, respectively; F(1, 22)=28.539, p<0.001; Figure 1a). Responding on the inactive lever during these sessions was minimal and there was no significant difference in inactive lever responses between the S+ and S− sessions (1.8±0.5 vs 1.3±0.5 responses per session, respectively; F(1, 22)=0.353, n.s.). Lever pressing behavior on the active lever was extinguished to 2.7±0.5 presses per session within 16–18 extinction sessions (Figure 1b). Subsequent presentation of the S− discriminative cue did not significantly alter behavior at the previously active lever as determined by two S− tests performed on the first and final days of reinstatement testing (F(2, 31)=0.079, n.s. Figure 1b). Responding on the inactive was also unaffected by S− presentation (F(2, 31)=2.214, n.s.). However, presentation of the S+ discriminative cue robustly increased nonreinforced lever pressing at the previously active lever to 14.2±4.0 presses per session which was significantly different from behavior elicited under either extinction (F(1, 20)=9.832, p<0.005) or S− (F(1, 20)=10.21, p<0.005) conditions. Presentation of the S+ did not alter responding on the inactive lever relative to either extinction (F(1, 20)=3.097, n.s.) or S− (F(1, 20)=0.31, n.s.) conditions. Intra-NAC vehicle administration resulted in a slight but nonsignificant reduction in S+-induced heroin-seeking behavior (F(1, 20)=1.230), and did not alter responding on the inactive lever (F(1, 20)=3.66, n.s.). Intra-NAC SR 141716A pretreatment produced a dose-dependent reduction in S+-induced heroin seeking as compared with intra-NAC vehicle (F(2, 33)=4.505, p<0.05), with significant reductions observed following 0.3 and 3 g per side infusions. Intra-NAC SR 141716A pretreatment did not alter responding on the inactive lever as compared with intra-NAC vehicle (F(2, 33)=0.255, n.s.). Subsequent tests were performed to evaluate the effect of intraperitoneal SR 1417161A pretreatment on S+-induced heroin seeking (Figure 1c). There was no significant effect of intraperitoneal vehicle administration on S+-induced heroin seeking (F(1, 17)=0.946) or responding at the inactive lever (F(1, 17)=0.228), n.s.). However, heroin seeking was significantly reduced following 3 mg/kg SR 141716A (i.p.) as compared with vehicle pretreatment (F(1, 16)=14.399, p<0.005). Intraperitoneal SR 141716A did not alter responding at the inactive lever as compared with vehicle pretreatment (F(1, 16)=2.286, n.s.). As shown in Figure 2, drug infusion sites were found to be placed in the core subregion of the NAC.
Effect of intra-NAC SR 141716A infusions on cue-induced reinstatement of heroin-seeking behavior (n=12). Panel a shows lever pressing on the drug-paired lever during the final 3 days of discrimination training. Data from both heroin self-administration (including presentation of the drug-paired discriminative cue (S+)) and saline self-administration (including presentation of the nondrug discriminative cue (S−)) are shown. Following extinction of lever pressing behavior the effects of conditioned cue exposure were evaluated (panel b). Presentation of the saline-associated cue (S−) did not elicit lever-pressing behavior. However, presentation of the heroin-associated cue (S+) induced a robust increase in nonreinforced lever pressing, or drug-seeking behavior. Cue-induced drug-seeking behavior was dose-dependently reduced by intra-NAC infusions of the CB1 receptor antagonist SR 141716A (p<0.05), and both the 0.3 and 3 μg doses significantly attenuated cue-induced drug seeking as compared with vehicle infusions. Subsequently, the effect of intra-peritoneal SR 141716A administration on cue-induced drug seeking was evaluated (panel c). As previously observed by others, peripherally administered SR 141716A significantly reduced cue-induced heroin-seeking behavior (p<0.005). ††p<0.001 vs responding under either extinction or S− conditions. *p<0.05 vs vehicle pretreatment. **p<0.005 vs vehicle pretreatment.
Schematic representations of bilateral infusion cannula placements within the nucleus accumbens (n=12). Plate shown is 1.7 mm anterior to bregma (adapted from Paxinos and Watson, 1998).
Intra-PFC SR 141716A Infusion Dose-Dependently Decreases Cue-Induced Heroin-Seeking Behavior
During the final 3 days of cue discrimination training rats in the PFC group (n=8) elicited significantly greater lever pressing on the active lever during the S+ heroin sessions than during the S− saline sessions (8.20±0.6 vs 2.0±0.5 lever presses per session, respectively; F(1, 14)=12.732, p<0.005; Figure 3a). Responding on the inactive lever during these sessions was minimal and there was no significant difference in inactive lever responses between the S+ and S− sessions (1.0±0.6 vs 2.1±1.3 responses per session, respectively; F(1, 14)=0.732, n.s.). Lever pressing behavior on the active lever was extinguished to 2.0±0.3 presses per session within 16–18 extinction sessions (Figure 3b). Subsequent presentation of the S− discriminative cue did not significantly alter behavior at the previously active lever (F(2, 19)=0.878, n.s.) or at the inactive lever (F(2, 19)=2.704, n.s.) as determined by two S− tests performed on the first and final days of reinstatement testing (F(2, 19)=0.878, n.s.). However, presentation of the S+ discriminative cue robustly increased nonreinforced lever pressing at the previously active lever to 11.4±2.6 presses per session which was significantly different from behavior elicited under either extinction (F(1, 14)=12.732, p<0.005) or S− (F(1, 14=10.20, p<0.01) conditions. Responding at the inactive lever was not significantly affected by S+ presentation compared with either extinction (F(1, 14)=0.436, n.s.) or S− (F(1, 14)=1.487, n.s.) conditions. There was no significant effect of intra-PFC vehicle infusion on S+-induced heroin-seeking behavior (F(1, 14)=0.011) or responding at the inactive lever (F(1, 14)=0.252, n.s.). However, intra-PFC SR 141716A pretreatment produced a dose-dependent reduction in S+-induced heroin seeking as compared with intra-PFC vehicle (F(2, 21)=3.718, p<0.05), with significant reductions observed following the 3 μg per side infusion. There were no significant effects of intra-PFC SR 141716A on responding at the inactive lever as compared with vehicle (F(2, 21)=0.171). Subsequent tests were performed to evaluate the effect of intraperitoneal SR 1417161A pretreatment on S+-induced heroin seeking (Figure 3c). There was no significant effect of intraperitoneal vehicle administration on S+-induced heroin seeking (F(1, 12)=0.923)) or responding at the inactive lever (F(1, 17)=0.228, n.s.). However, heroin seeking was significantly reduced following 3 mg/kg SR 141716A (i.p.) as compared with vehicle pretreatment (F(1, 10)=15.81, p<0.005). Intraperitoneal SR 141716A did not alter responding at the inactive lever as compared with vehicle pretreatment (F(1, 16)=2.286, n.s.). Drug infusion placements in the PFC are shown schematically in Figure 4.
Effect of intra-PFC SR 141716A infusions on cue-induced reinstatement of heroin-seeking behavior (n=8). Panel a shows lever pressing on the drug-paired lever during the final 3 days of discrimination training. Data from both heroin self-administration and saline self-administration are shown. Following extinction of lever pressing behavior the presentation of the heroin cue (S+) induced a robust increase in nonreinforced heroin-seeking behavior (Panel b). This effect was dose-dependently reduced by intra-PFC infusions of SR 141716A (p<0.05), with the 3 μg dose significantly attenuating cue-induced drug-seeking as compared with vehicle infusions. A similar blockade of cue-induced drug-seeking was observed following intraperitoneal SR 141716A administration (p<0.005; panel c). ††p<0.001 vs extinction or S− conditions. *p<0.05 vs vehicle. **p<0.005 vs vehicle.
Schematic representations of the bilateral infusion cannula placements within the prefrontal cortex (n=8). Plate shown is 3.0 mm anterior to bregma (adapted from Paxinos and Watson, 1998).
Intra-BLA SR 141716A Infusion Does Not Alter Cue-Induced Heroin-Seeking Behavior
During the final 3 days of cue discrimination training rats in the BLA group (n=9) elicited significantly greater lever pressing on the active lever during the S+ heroin sessions than during the S− saline sessions (11.8±1.0 vs 2.1±0.7 lever presses per session, respectively; F(1, 16)=49.869, p<0.0001; Figure 5a). Responding on the inactive lever during these sessions was minimal and there was no significant difference in inactive lever responses between the S+ and S− sessions (3.6±2.6 vs 2.2±0.7 responses per session, respectively; F(1, 16)=0.239). Lever pressing behavior on the active lever was extinguished to 2.6±0.6 presses per session within 16–18 extinction sessions (Figure 5b). Subsequent presentation of the S− discriminative cue did not significantly alter behavior at the previously active lever as determined by two S− tests performed on the first and final days of reinstatement testing (F(2, 24)=0.772). Responding at the inactive lever was also unaffected by S− presentation (F(2, 24)=0.102, n.s.). However, presentation of the S+ discriminative cue robustly increased nonreinforced lever pressing at the previously active lever to 16.8±1.5 presses per session which was significantly different from behavior elicited under either extinction (F(1, 16)=78.392, p<0.0001) or S− (F(1, 16)=56.045, p<0.0001) conditions. Presentation of the S+ did not alter responding at the inactive lever relative to either extinction (F(1, 16)=0.812, n.s.) or S− (F(1, 16)=2.352, n.s.). Intra-BLA vehicle infusion significantly reduced S+-induced heroin-seeking behavior (F(1, 16)=7.0; p<0.05) but did not alter responding at the inactive lever (F(1, 16)=0.048, n.s.). However, intra-BLA SR 141716A pretreatment did not significantly alter S+-induced heroin seeking relative to levels observed following intra-BLA vehicle (F(2, 24)=0.324) and did not alter responding at the inactive lever (F(2, 24)=0.258, n.s.). Subsequent tests were performed to evaluate the effect of intraperitoneal SR 1417161A pretreatment on S+-induced heroin seeking (Figure 5c). There was no significant effect of intraperitoneal vehicle administration on S+-induced heroin seeking (F(1, 16)=3.234) or responding at the inactive lever (F(1, 16)=0.603, n.s.). However, in contrast to the lack of effects observed following intra-BLA SR 141716A administration, heroin seeking was significantly reduced following intraperitoneal SR 141716A as compared with vehicle pretreatment (F(1, 16)=5.894, p<0.05). Intraperitoneal SR 141716A did not alter responding at the inactive lever as compared with vehicle pretreatment (F(1, 16)=0.026, n.s.). Drug infusion placements in the BLA are shown schematically in Figure 6.
Effect of intra-BLA SR 141716A infusions on cue-induced reinstatement of heroin-seeking behavior (n=9). Panel a shows lever pressing on the drug-paired lever during the final 3 days of discrimination training. Data from both heroin self-administration and saline self-administration are shown. Following extinction of lever pressing behavior the presentation of the heroin cue (S+) induced a robust increase in nonreinforced heroin-seeking behavior (panel b). This effect was unaltered by intra-BLA infusions of SR 141716A (n.s.). In contrast, cue-induced drug-seeking behavior in these animals was significantly attenuated by intraperitoneally administered SR 141716A (p<0.05; panel c). ††p<0.001 vs extinction or S− conditions. *p<0.05 vs vehicle.
Schematic representations of the bilateral infusion cannula placements within the basolateral amygdala (n=9). Plate shown is 2.56 mm posterior to bregma (adapted from Paxinos and Watson, 1998).
Repeated Exposure to the S+ Does Not Diminish the Reinstatement of Drug-Seeking Behavior
To evaluate whether repeated S+ exposure diminishes the reinstatement of drug-seeking behavior, 15 animals were given a second pretreatment-free evaluation of S+-induced reinstatement following completion of all tests with intracranial and systemic SR 141716A. Thus in these animals the reinstatement of extinguished drug-seeking behavior was compared between the first S+ exposure and the seventh S+ exposure (five S+ exposures occurred within the course of the intracranial and systemic SR 141716A tests). In this grouping of animals the initial S+ presentation elicited 15.73±2.52 responses on the drug-associated lever, and the seventh S+ presentation elicited 14.13±2.6 responses on the drug-associated lever. There was no significant difference in S+-induced drug seeking between these two tests (F(1, 28)=0.194, n.s.). This indicates that under the present conditions the relative response to S+ presentation did not diminish with repeated testing, supporting the conclusion that the effects observed with SR 1417116A are not biased by a diminished motivational effect of S+ presentation from repeated exposure.
DISCUSSION
Our results demonstrate that local administration of the CB1 receptor antagonist SR 141716A into the NAC and PFC, but not BLA, dose-dependently decreases cue-induced reinstatement of heroin-seeking behavior. In general, these findings suggest an involvement of cannabinoid CB1 receptors in the NAC and PFC in mediating cue-induced relapse to drug-seeking behavior. In the present study, systemic administration of SR 141716A reduced cue-induced reinstatement of heroin-seeking behavior confirming previous observations suggesting a role for the CB1 receptor in the modulation of cue-induced heroin relapse (De Vries et al, 2003). In addition, we observed that intra-PFC infusions attenuate cue-induced reinstatement of heroin-seeking behavior. These results are in agreement with present knowledge suggesting the PFC as a neural substrate underlying drug-seeking behavior. For example, brain-imaging studies in humans have shown that cue-induced cocaine craving is associated with activation of the PFC (Childress et al, 1999; Kilts et al, 2001). Similar findings have been observed in abstinent opioid-dependent subjects when drug-related stimuli are presented (Daglish et al, 2001). Studies with animals further support a role for the PFC in the mediation of relapse of drug seeking. For example, cue-induced reinstatement of heroin-seeking but not sucrose-seeking behavior is accompanied by enhanced expression of immediate-early genes (IEG) associated with gene transcription and neuronal signaling in the PFC (Koya et al, 2006) suggesting that this brain region is specifically activated in response to stimuli predicting the availability of heroin.
Examination of the potential role of the PFC in relapse of drug seeking has also revealed some of the neuropharmacological mechanisms through which conditioned stimuli induce drug-seeking behavior. For example, inactivation of PFC glutamate output neurons prevents both drug- and stress-induced reinstatement of cocaine-seeking behavior (McFarland et al, 2003, 2004; McLaughlin and See, 2003) by preventing increased NAC glutamate levels associated with both types of reinstatement (McFarland et al, 2003, 2004). In addition, stimulation of GABAA receptors in the PFC blocks the expression of conditioned hyperactivity in a cocaine-paired environment (Franklin and Druhan, 2000). More recently, Laviolette and Grace (2006) showed that the PFC modulates associative learning in an olfactory-fear conditioning task via an eCB mechanism. In the present study, we show that blockade of PFC CB1 receptors reduces cue-induced reinstatement of heroin-seeking behavior. These findings suggest that cannabinoid signaling in the PFC modulates the salience of discriminative stimuli (S+) signaling heroin availability.
Although the neurochemical mechanisms through which PFC CB1 receptors regulate relapse of heroin-seeking behavior were not evaluated in the present study, it is possible that CB1 receptors modulate the response of PFC output neurons through an inhibitory control over GABA release, as observed in the hippocampus (Katona et al, 1999). In tandem, it has been reported that GABA manipulations in the PFC modify relapse of drug-seeking behavior (McFarland et al, 2003, 2004; McLaughlin and See, 2003). Thus, it is possible that intra-PFC administration of SR 141716A reduces cue-induced excitation of PFC pyramidal cells through its action on CB1 receptors located on local GABAergic interneurons. This mechanism is further supported by data showing that CB1 receptor agonists increase PFC pyramidal cell activity (Pistis et al, 2001). Alternatively, a dopamine-dependent mechanism for the actions of SR 141716A on cue-induced heroin reinstatement is also possible based on the observation that administration of SR 141716A selectively increases dopamine release in the PFC (Tzavara et al, 2003) and dopaminergic activation of GABAergic interneurons appears to mediate inhibition of pyramidal cells in the PFC (Pirot et al, 1992; Sesack and Bunney, 1989; Sesack et al, 1995a, 1995b; Gioanni et al, 1998). However, it is unknown whether this dopaminergic effect is modulated by CB1 receptors in the PFC or elsewhere. It is worth noting that many of the studies cited above have evaluated mechanisms throughout the infralimbic and more dorsal prelimbic divisions of the PFC, while the injector in the present study terminated predominantly in the infralimbic cortex. Further experiments are required to evaluate potential differences in CB1-mediated effects between the infralimbic and prelimbic cortices and to determine the neurochemical mechanisms through which CB1 receptors in the PFC modulate cue-induce reinstatement of heroin-seeking behavior.
With regard to the NAC, it has been demonstrated that this brain region is involved in the control of goal-directed behaviors such as Pavlovian appetitive conditioning (Parkinson et al, 1999; Di Ciano et al, 2001). In the context of drug addiction, the NAC core plays a similar role mediating the expression of motor behaviors in response to drug-predictive stimuli presumably encoded by the PFC (Di Ciano and Everitt, 2001). In agreement, a distinct pattern of firing is observed in NAC cells during presentation of conditioned stimuli (Carelli, 2000; Ghitza et al, 2003; Nicola et al, 2004; Yun et al, 2004), an effect that persists after an extended period of cocaine abstinence (Hollander and Carelli, 2007). Pharmacological inactivation of the NAC core blocks both cocaine-induced reinstatement of drug seeking (Cornish and Kalivas, 2000) and acquisition of a second-order schedule of heroin reinforcement, consistent with impairments in drug-seeking behavior (Hutcheson et al, 2001). Cue-induced reinstatement of heroin-seeking behavior is accompanied by alterations in the expression of synaptic plasticity-related (Lee et al, 2004) and intracellular signaling-related (Yao et al, 2005) genes in NAC cells suggesting this brain region might be also involved in the development of heroin-seeking behavior. Recent evidence demonstrates that intra-NAC administration of the CB1 receptor antagonist AM251 inhibits cocaine-induced reinstatement (Xi et al, 2006). We show that both systemic and intra-NAC SR 141716A infusions reduce cue-induced reinstatement of heroin-seeking behavior. Our results are in agreement with previous studies describing a role for the NAC in drug-seeking behavior and provide further evidence for an eCB modulation within the NAC on cue-induced reinstatement of drug seeking. Although further studies are required to elucidate the neurochemical mechanisms through which NAC CB1 receptors modulate reinstatement of heroin-seeking behavior, it is possible that SR 141716A administration decreases cue-induced reinstatement of heroin-seeking behavior by preventing CB1-mediated inhibition of local GABAergic interneurons. This is based on evidence showing that CB1 receptor activation decreases GABA release in the NAC (Hoffman and Lupica, 2001; Manzoni and Bockaert, 2001). In addition, it is possible that CB1 receptors modulate reinstatement of heroin-seeking behavior through regulation of glutamate release in the NAC since CB1 receptors are also located on glutamatergic afferents in the NAC (Mailleux and Vanderhaeghen, 1992) and CB1 receptor blockade has been shown to attenuate cocaine-primed relapse behavior through a glutamatergic mechanism in the NAC (Xi et al, 2006).
An important role for the BLA in drug-seeking behavior has been established in studies showing that electrical stimulation of the BLA reinstates lever pressing for cocaine after extinction (Hayes et al, 2003) and that excitotoxic lesion (Meil and See, 1997; Yun and Fields, 2003) and reversible inactivation (Grimm and See, 2000) of this brain region attenuates cue-induced reinstatement of cocaine-seeking behavior. Evidence also suggests that alterations in the BLA dopamine system are associated with the expression of cocaine-seeking behavior. For example, cue-induced relapse of cocaine-seeking behavior is associated with increased dopamine release in the BLA (Weiss et al, 2000) and is attenuated by blockade of dopamine but not glutamate receptors in the BLA (See et al, 2001; Di Ciano and Everitt, 2004). These results are further supported by observations that intra-BLA infusions of a nonspecific dopamine agonist potentiate cocaine-seeking behavior (Ledford et al, 2003) and that intra-BLA administration of D1 dopamine receptor antagonists reverses cue-induced cocaine relapse and the corresponding increase in c-fos expression, a marker for neural activation (Ciccocioppo et al, 2001b). Although limited, there is also evidence suggesting that the integrity of the BLA is necessary for cue-induced reinstatement of heroin-seeking behavior (Fuchs and See, 2002).
We expected to observe a reduction in cue-induced reinstatement by blocking CB1 receptors in the BLA since these receptors are highly expressed by CCK-positive GABAergic interneurons in the BLA (Katona et al, 2001) that control the excitation of BLA output neurons (Collins and Pare, 1999). It was anticipated that CB1 receptor blockade would serve to maintain inhibitory GABAergic tone (Hoffman and Lupica, 2001; Manzoni and Bockaert, 2001) thereby decreasing the excitability of BLA neurons involved in the expression of drug-stimuli associations evoking relapse of heroin seeking. However, our results show that SR 141716A administration in the BLA does not affect cue-induced reinstatement of heroin-seeking behavior, even though intraperitoneal SR 141716A injection in this group of rats blocked cue-induced reinstatement. The lack of effect of intra-BLA SR 141716A on cue-induced heroin-seeking behavior does not necessarily preclude an involvement of BLA CB1 receptors in the modulation of cue-induced reinstatement of heroin seeking. It is possible that CB1 receptor signaling in the BLA does not participate in the expression of cue-induced reinstatement of heroin seeking, but rather is involved in the formation of associations between cues and drug availability. This rationale is based on evidence that the BLA performs initial encoding of stimulus associations, which are then transferred to the PFC for long-term storage. For example, single neurons in the PFC that receive orthodromic inputs from the BLA require an active input from the BLA in order to encode emotional memories (Laviolette et al, 2005). Once emotional associative learning has occurred at the level of the single PFC neuron, inactivation of the BLA has no effect on the expression of this learning (Laviolette and Grace, 2006). Thus, it is possible that after the initial encoding of emotional associations have taken place within the BLA, this information is then transferred to the PFC for long-term storage. Based on this construct, it is conceivable that CB1 receptors in the BLA participate in the initial encoding of stimulus-reward associations, but not in the subsequent expression of cue-induced drug seeking.
Overall, little is known regarding to the role of CB1 receptors in the PFC, NAC, and BLA in the modulation of drug-seeking behavior. Xi et al (2006) showed that intra-NAC perfusion of the CB1 receptor antagonist AM251 inhibits cocaine-primed reinstatement of cocaine-seeking behavior and cocaine-induced increases in NAC glutamate. In addition, SR 141716A infusions into the NAC shell, BLA, and PFC dose-dependently reduce nicotine-seeking behavior (Kodas et al, 2007) suggesting that BLA CB1 receptors may be involved in the relapse behavior associated with other drugs of abuse and that other brain subregions such as the NAC may also be involved in cue-induced reinstatement. Thus, we cannot rule out the possibility that our results have involved the diffusion of SR 141716A to other relevant brain subregions such as the NAC shell. Recent evidence demonstrates that heroin self-administration in rats is accompanied by increases in CB1 receptor binding and/or functionality in brain regions such as the PFC, NAC, and amygdala (Fattore et al, 2007). In this study, simultaneous increases in CB1 receptor density and agonist-induced GTPγS binding were observed in the amygdala suggesting that voluntary heroin intake is associated with adaptive changes in CB1 receptor expression in a brain region associated with the formation of associative learning. It is unknown, however, whether similar changes in CB1 receptor expression and function are present several weeks after heroin exposure, and whether such functional changes play a role in the induction of drug-seeking behavior.
In conclusion, the present study provides pharmacological evidence that CB1 receptors in the NAC, PFC, but not BLA are involved in mediating cue-induced reinstatement of heroin-seeking behavior. Further experiments are required to determine the neurochemical mechanisms through which CB1 receptors in the PFC and NAC modulate cue-induced reinstatement of heroin-seeking behavior and to clarify the potential role of CB1 receptors in the BLA in the establishment of drug-stimulus associations that contribute to relapse behavior.
References
Caillé S, Parsons LH (2006). Cannabinoid modulation of opiate reinforcement through the ventral striatopallidal pathway. Neuropsychopharmacology 31: 804–813.
Carelli RM (2000). Activation of accumbens cell firing by stimuli associated with cocaine delivery during self-administration. Synapse 35: 238–242.
Carter BL, Tiffany ST (1999). Meta-analysis of cue-reactivity in addiction research. Addiction 94: 327–340.
Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP (1999). Limbic activation during cue-induced cocaine craving. Am J Psychiatry 156: 11–18.
Ciccocioppo R, Angeletti S, Weiss F (2001a). Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res 25: 1414–1419.
Ciccocioppo R, Sanna PP, Weiss F (2001b). Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D(1) antagonists. Proc Natl Acad Sci USA 98: 1976–1981.
Collins DR, Pare D (1999). Reciprocal changes in the firing probability of lateral and central medial amygdala neurons. J Neurosci 19: 836–844.
Cornish JL, Kalivas PW (2000). Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci 20: RC89.
Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S et al (2001). Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry 158: 1680–1686.
DeJong W (1994). Relapse prevention: an emerging technology for promoting long-term drug abstinence. Int J Addict 29: 681–705.
De Vries TJ, de Vries W, Janssen MCW, Schoffelmeer ANM (2005). Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav Brain Res 161: 164–168.
De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN (2003). Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 168: 164–169.
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J et al (2001). A cannabinoid mechanism in relapse to cocaine seeking. Nat Medicine 7: 1151–1154.
Di Ciano P, Cardinal RN, Cowell RA, Little SJ, Everitt BJ (2001). Differential involvement of NMDA, AMPA/kainate, and dopamine receptors in the nucleus accumbens core in the acquisition and performance of pavlovian approach behavior. J Neurosci 21: 9471–9477.
Di Ciano P, Everitt BJ (2001). Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine seeking behavior. Neuropsychopharmacology 25: 341–360.
Di Ciano P, Everitt BJ (2004). Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J Neurosci 24: 7167–7173.
Egertová M, Elphick MR (2000). Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol 422: 159–171.
Epstein DH, Preston KL, Stewart J, Shaham Y (2006). Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189: 1–16.
Fattore L, Spano MS, Cossu G, Deiana S, Fratta W (2003). Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur J Neurosci 17: 1723–1726.
Fattore L, Vigano D, Fadda P, Rubino T, Fratta W, Parolaro D (2007). Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur J Neurosci 25: 2191–2200.
Franklin TR, Druhan JP (2000). Involvement of the nucleus accumbens and medial prefrontal cortex in the expression of conditioned hyperactivity to a cocaine-associated environment in rats. Neuropsychopharmacology 23: 633–644.
Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE (2005). The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology 30: 296–309.
Fuchs RA, See RE (2002). Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharmacology (Berl) 160: 425–433.
Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO (2003). Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci 23: 7239–7245.
Gioanni Y, Thierry AM, Glowinski J, Tassin JP (1998). Alpha1-adrenergic, D1, and D2 receptors interactions in the prefrontal cortex: implications for the modality of action of different types of neuroleptics. Synapse 30: 362–370.
Gracy KN, Dankiewicz LA, Weiss F, Koob GF (2000). Heroin-specific stimuli reinstate operant heroin-seeking behavior in rats after prolonged extinction. Pharm Biochem Behav 65: 489–494.
Grimm JW, See RE (2000). Dissociation of primary and secondary reward-relevant limbic nuclei in an animal model of relapse. Neuropsychopharmacology 22: 473–479.
Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL (2003). Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology (Berl) 168: 75–83.
Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro auto-radiographic study. J Neurosci 11: 563–583.
Hoffman AF, Lupica CR (2001). Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol 85: 72–83.
Hollander JA, Carelli RM (2007). Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci 27: 3535–3539.
Hutcheson DM, Parkinson JA, Robbins TW, Everitt BJ (2001). The effects of nucleus accumbens core and shell lesions on intravenous heroin self administration and the acquisition of drug-seeking behaviour under a second-order schedule of heroin reinforcement. Psychopharmacology (Berl) 153: 464–472.
Jaffe JH, Cascella NG, Kumor KM, Sherer MA (1989). Cocaine-induced cocaine craving. Psychopharmacology (Berl) 97: 59–64.
Kalivas PW, Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Katona I, Rancz EA, Acsády L, Ledent C, Mackie K, Hájos N et al (2001). Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci 21: 9506–9518.
Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K et al (1999). Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558.
Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F et al (2001). Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58: 334–341.
Kodas E, Cohen C, Louis C, Griebel G (2007). Cortico-limbic circuitry for conditioned nicotine-seeking behavior in rats involves endocannabinoid signaling. Psychopharmacology (Berl) 194: 161–171.
Koya E, Spijker S, Voorn P, Binnekade R, Schmidt ED, Schoffelmeer ANM et al (2006). Enhanced cortical and accumbal molecular reactivity associated with conditioned heroin, but not sucrose-seeking behaviour. J Neurochem 98: 905–915.
Kreek MJ, Koob GF (1998). Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend 51: 23–47.
Laviolette SR, Grace AA (2006). Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci 26: 6458–6468.
Laviolette SR, Lipski WJ, Grace AA (2005). A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci 25: 6066–6075.
Ledford CC, Fuchs RA, See RE (2003). Potentiated reinstatement of cocaine-seeking behavior following D-amphetamine infusion into the basolateral amygdala. Neuropsychopharmacology 28: 1721–1729.
Lee JL, Everitt BJ, Thomas KL (2004). Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304: 839–843.
Leshner AI (1997). Addiction is a brain disease, and it matters. Science 278: 5335–5345.
Mailleux P, Vanderhaeghen JJ (1992). Localization of cannabinoid receptor in the human developing and adult basal ganglia. Higher levels in the striatonigral neurons. Neurosci Lett 148: 173–176.
Manzoni OJ, Bockaert J (2001). Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharmacol 412: R3–R5.
Matsuda LA, Bonner TI, Lolait SJ (1993). Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol 327: 535–550.
McDonald AJ, Mascagni F (2001). Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience 107: 641–652.
McFarland K, Davidge SB, Lapish CC, Kalivas P (2004). Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24: 1551–1560.
McFarland K, Lapish CC, Kalivas P (2003). Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23: 3531–3537.
McLaughlin J, See RE (2003). Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology 168: 57–65.
McLellan AT, Lewis DC, O'Brien CP, Kleber HD (2000). Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284: 1689–1695.
Meil WM, See RE (1997). Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res 87: 139–148.
Nicola SM, Yun IA, Wakabayashi KT, Fields HL (2004). Cue-evoked firing of nucleus accumbens neurons encodes motivational significance during a discriminative stimulus task. J Neurophysiol 91: 1840–1865.
O'Brien CP, Childress AR, McLellan AT, Ehrman R (1992). Classical conditioning in drug-dependent humans. Ann NY Acad Sci 654: 400–415.
Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ (1999). Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J Neurosci 19: 2401–2411.
Paxinos G, Watson C (1998). The Rat Brain in Stereotaxic Coordinates. Academic Press: San Diego.
Pirot S, Godbout R, Mantz J, Tassin JP, Glowinski J, Thierry AM (1992). Inhibitory effects of ventral tegmental area stimulation on the activity of prefrontal cortical neurons: evidence for the involvement of both dopaminergic and GABAergic components. Neuroscience 49: 857–865.
Pistis M, Porcu G, Melis M, Diana M, Gessa GL (2001). Effects of cannabinoids on prefrontal neuronal responses to ventral tegmental area stimulation. Eur J Neurosci 14: 96–102.
See RE (2005). Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol 526: 140–146.
See RE, Kruzich PJ, Grimm JW (2001). Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharmacology (Berl) 154: 301–310.
Serra S, Brunetti G, Pani M, Vacca G, Carai MAM, Gessa GL et al (2002). Blockade by the cannabinoid CB(1) receptor antagonist, SR 141716, of alcohol deprivation effect in alcohol-preferring rats. Eur J Pharmacol 443: 95–97.
Sesack SR, Bressler CN, Lewis DA (1995a). Ultrastructural associations between dopamine terminals and local circuit neurons in the monkey prefrontal cortex: a study of calretinin-immunoreactive cells. Neurosci Lett 200: 9–12.
Sesack SR, Bunney BS (1989). Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J Pharmacol Exp Ther 248: 1323–1333.
Sesack SR, Snyder CL, Lewis DA (1995b). Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol 363: 264–280.
Shaham Y, Erb S, Stewart J (2000). Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Rev 33: 13–33.
Shalev U, Grimm JW, Shaham Y (2002). Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54: 1–42.
Sinha R, Catapano D, O'Malley S (1999). Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology 142: 343–351.
Stewart J (2000). Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug taking. J Psychiatry Neurosci 25: 125–136.
Tims FM, Leukefeld CG (1986). Relapse and recovery in drug abuse. NIDA Research Monograph 72: 1–195.
Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM (1998). Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83: 393–411.
Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP et al (2003). The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol 138: 544–553.
Weiss F (2005). Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 5: 9–19.
Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O (2000). Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci USA 97: 4321–4326.
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL (2006). Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci 26: 8531–8536.
Yao L, McFarland K, Fan P, Jiang Z, Inoue Y, Diamond I (2005). Activator of G protein signaling 3 regulates opiate activation of protein kinase A signaling and relapse of heroin-seeking behavior. Proc Natl Acad Sci USA 102: 8746–8751.
Yun IA, Fields HL (2003). Basolateral amygdala lesions impair both cue- and cocaine-induced reinstatement in animals trained on a discriminative stimulus task. Neuroscience 121: 747–757.
Yun IA, Nicola SM, Fields HL (2004). Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci 20: 249–263.
Zavala AR, Biswas S, Harlan RE, Neisewander JL (2007). Fos and glutamate AMPA receptor subunit co-expression associated with cue-elicited cocaine-seeking behavior in abstinent rats. Neuroscience 145: 438–452.
Acknowledgements
This study was supported by NIDA grant R21 DA019962 and NIAAA grant RO1 AA014619 (LHP). We gratefully acknowledge the generous gift of SR 141716A provided by the National Institute of Mental Health Chemical Synthesis and drug Supply Program. This is publication 19004 from the Scripps Research Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
DISCLOSURE/CONFLICT OF INTEREST
The authors listed on this manuscript (L Alvarez-Jaimes, I Polis, and LH Parsons) do not have any potential conflicts of interest related to the subject of this report. Further, each of the authors on this manuscript is supported in full by NIH research grants and have not received, and do not anticipate receiving, any compensation for professional services from any source outside of the NIH.
Rights and permissions
About this article
Cite this article
Alvarez-Jaimes, L., Polis, I. & Parsons, L. Attenuation of Cue-Induced Heroin-Seeking Behavior by Cannabinoid CB1 Antagonist Infusions into the Nucleus Accumbens Core and Prefrontal Cortex, but Not Basolateral Amygdala. Neuropsychopharmacol 33, 2483–2493 (2008). https://doi.org/10.1038/sj.npp.1301630
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301630
Keywords
This article is cited by
-
Non-Opioid Treatments for Opioid Use Disorder: Rationales and Data to Date
Drugs (2020)
-
Relapse to opioid seeking in rat models: behavior, pharmacology and circuits
Neuropsychopharmacology (2019)
-
Cannabinoid CB1 receptor neutral antagonist AM4113 inhibits heroin self-administration without depressive side effects in rats
Acta Pharmacologica Sinica (2019)
-
Role of prefrontal cortex in the extinction of drug memories
Psychopharmacology (2019)
-
Effects of menthol and its interaction with nicotine-conditioned cue on nicotine-seeking behavior in rats
Psychopharmacology (2017)