Abstract
The neuropeptide galanin coexists with norepinephrine and serotonin in neural systems mediating emotion. Previous findings suggested that galanin modulates anxiety-related behaviors in rodents. Three galanin receptor subtypes have been cloned; however, understanding their functions has been limited by the lack of galanin receptor subtype-selective ligands. To study the role of the galanin GAL-R1 receptor subtype in mediating anxiety-related behavior, we generated mice with a null mutation in the Galr1 gene. GAL-R1 −/− are viable and show no abnormalities in health, neurological reflexes, motoric functions, or sensory abilities. On a battery of tests for anxiety-like behavior, GAL-R1 −/− showed increased anxiety-like behavior on the elevated plus-maze test. Anxiety-related behaviors on the light/dark exploration, emergence, and open field tests were normal in GAL-R1 −/−. This test-specific anxiety-like phenotype was confirmed in a second, independent cohort of GAL-R1 null mutant mice and +/+ controls. Principal components factor analysis of behavioral scores from 279 mice suggested that anxiety-like behavior on the elevated plus-maze was qualitatively distinct from behavior on other tests in the battery. In addition, exposure to the elevated plus-maze produced a significantly greater neuroendocrine response than exposure to the light/dark exploration test, as analyzed in normal C57BL/6J mice. These behavioral findings in the first galanin receptor null mutant mouse are consistent with the hypothesis that galanin exerts anxiolytic actions via the GAL-R1 receptor under conditions of relatively high stress.
Similar content being viewed by others
INTRODUCTION
Galanin is a 29 amino-acid neuropeptide involved in the regulation of multiple behaviors, including feeding, cognition, seizures, sexual behavior, nociception, and emotion (for reviews, see Hökfelt et al, 1998a; Mazarati et al, 2001; Wrenn and Crawley, 2001). In most cases, galanin acts as an inhibitory modulator, hyperpolarizing neurons and inhibiting the release of norepinephrine, serotonin, acetylcholine, and glutamate (Seutin et al, 1989; Pieribone et al, 1995; Xu et al, 1998a,1998b; Kehr et al, 2002). Galanin coexists with norepinephrine in 80–90% of rodent locus coeruleus neurons, and with serotonin in 30% of rat dorsal raphe neurons (Skofitsch and Jacobowitz, 1986; Melander et al, 1986; Holets et al, 1988; Merchenthaler et al, 1993; Hökfelt et al, 1998b; Perez et al, 2001). Galanin and its receptors are also found in cortico-limbic regions implicated in emotional behavior, including the amygdala, hippocampus, septum, and hypothalamus (for reviews, see Iismaa and Shine, 1999; Branchek et al, 2000; Waters and Krause, 2000). This pattern of distribution suggests that galanin may mediate affective states (Hökfelt et al, 1999; Wrenn and Crawley, 2001; Holmes et al, 2002b).
Galanin has striking effects on neural, neuroendocrine, and sympathetic responses to stress (Carey et al, 1993; Holmes and Crawley, 1995). For example, increases in plasma ACTH following exposure to ether stress in rats were attenuated by galanin administered directly into the hypothalamus (Hooi et al, 1990), but potentiated by galanin administered peripherally (Malendowicz et al, 1994). Galanin increased the sympathetic response to social stress in rats (Ceresini et al, 1998). In human subjects, galanin inhibited sympathetic outflow (ie increased plasma norepinephrine) in response to hypoglycemia (degli Uberti et al, 1995b) and to upright posture-induced hypotension (degli Uberti et al, 1995a). Further, exposure to chronic stress has been shown to increase galanin gene expression in rats. Repeated restraint stress produced significant increases in prepro-galanin mRNA expression in the hypothalamus (Makino et al, 1999) and amygdala (Sweerts et al, 1999; Palkovits, 2000), as well as significant decreases in galanin receptor density in the central and medial nuclei of the amygdala (Sweerts et al, 2000). Chronic exposure to other stressors, including social stress (Holmes et al, 1995) and repeated treadmill exercise (O'Neal et al, 2001) increased prepro-galanin mRNA expression in the locus coeruleus. In contrast, putatively less stressful manipulations, such as wheel running (Soares et al, 1999) and swim stress (Austin et al, 1990), failed to alter galanin gene expression. These findings have led to the hypothesis that galanin contributes to the homeostatic neural mechanisms mediating responses to repeated stress (Holmes and Crawley, 1995; O'Neal et al, 2001).
Behavioral studies support a role for galanin in anxiety-like behavior. Heilig and colleagues found that galanin administered intracerebroventricularly to rats produced an anxiolytic-like increase in punished responding for water in the Vogel conflict test, with no concomitant alteration in unpunished responding or nociception (Bing et al, 1993). The same laboratory subsequently showed that microinjections of galanin into the central nucleus of the amygdala, but not the parietal cortex, led to an anxiogenic-like suppression of punished responding in the Vogel conflict test, again without affecting control parameters of pain thresholds, locomotion, or unpunished responding (Moller et al, 1999). Interestingly, the same galanin treatment failed to alter anxiety-like behavior in the elevated plus-maze. The authors suggest that these test differences reflect a specific anxiety-related effect of galanin in tasks, such as the Vogel conflict test, that are highly stressful.
Recently, Morilak and co-workers demonstrated that galanin orchestrates the anxiety-like behavioral response to stress. Khoshbouei et al (2002b) found that both anxiety-like (elevated plus-maze, social interaction tests) and neuroendocrine (plasma adrenocorticotropin) responses to acute restraint stress in rats were blocked by administration of the nonspecific galanin receptor antagonist, M40, directly into the bed nucleus of the stria terminalis. In a separate study, Khoshbouei et al (2002a) found that rats subject to the combined stress of restraint and treatment with yohimbine, an α2 adrenergic autoreceptor antagonist that increases norepinephrine release in limbic regions, displayed a paradoxical decrease in anxiety-like behavior on the elevated plus-maze, as compared to either stressor alone. This anxiolytic-like response to high stress was found to be mediated by the release of galanin in the central nucleus of the amygdala and was blocked by intra-amygdala infusion of M40. In our laboratory, we have also found evidence that galanin exerts anxiolytic-like effects preferentially under conditions of high noradrenergic activity induced by yohimbine treatment, using a transgenic mouse model of constitutive galanin overexpression (Holmes et al, 2002b).
The specific galanin receptor subtype(s) underlying the effects of galanin on stress responses and anxiety-like behavior are unknown. Three G-protein-coupled galanin receptor subtypes (GAL-R1, GAL-R2, GAL-R3) have been cloned and found to show different, but partially overlapping, patterns of distribution in the central nervous system and periphery. The GAL-R1 receptor subtype has been cloned in human (Habert-Ortoli et al, 1994), rat (Parker et al, 1995), and mouse (Wang et al, 1997), and is expressed at moderate to high levels in the accessory olfactory tract, amygdala, hypothalamus, thalamus, hippocampus, spinal trigeminal nucleus, spinal cord, and dorsal root ganglia, but not peripheral tissues (Iismaa and Shine, 1999; O'Donnell et al, 1999; Branchek et al, 2000; Waters and Krause, 2000). GAL-R1 exerts it physiological actions in these regions via the activation of Gi/0 G-proteins, leading to inhibition of adenylyl cyclase and cAMP, and closure of voltage-sensitive calcium channels. Differentiating the functional roles of galanin receptor subtypes has been limited by the absence of subtype selective, high-affinity, soluble galanin receptor agonists and antagonists (Scott et al, 2000; Saar et al, 2002).
In the present study, we conducted behavioral phenotyping of a targeted GAL-R1 (Galr1) null mutant mouse to study the role of the galanin GAL-R1 receptor subtype in the mediation of anxiety-related behavior. To first exclude possible confounds on anxiety-related behaviors, GAL-R1 null mutant mice were compared to +/+ littermates on control measures of health, motor, neurological, and sensory functions. GAL-R1 mutants were assessed on a battery of tests for anxiety-related behaviors: elevated plus-maze, light/dark exploration, emergence, and open field. To determine whether these tests measured different forms of anxiety-like behavior that could be differentially sensitive to detecting an anxiety-like phenotype in GAL-R1 null mutant mice, we conducted a factor analysis of anxiety-related test scores from a large sample of mice. To examine whether exposure to these tests produced different neuroendocrine responses, plasma ACTH and corticosterone levels were measured after test exposure in normal C57BL/6J mice.
MATERIAL AND METHODS
Subjects
Galanin receptor GAL-R1 null mutant mice were generated as previously described (Jacoby et al, 2002a,2002b). 129Sv embryonic stem cells were microinjected into C57BL/6J blastocysts and backcrossed into C57BL/6J for five generations to avoid complications of a heterogeneous genetic background. Mice are viable, reproduce, and develop normally. RT-PCR analysis of GAL-R1 receptor mRNA levels in the brain and periphery has confirmed that the normal, full-length transcript encoding the GAL-R1 receptor is absent in GAL-R1 −/− mice (Jacoby et al, 2002a,2002b). Corresponding analysis of GAL-R1 +/− mice has not yet been conducted. Jacoby et al (2002a),(2002b) also found that GAL-R1 −/− mice bred and housed at the Garvan Institute of Medical Research (Sydney, Australia) show prominent spontaneous seizure activity. GAL-R1 null mutant mice and +/+ littermate controls from the original founder line generated at the Garvan Institute of Medical Research were rederived at The Jackson Laboratory (Bar Harbor, ME) and transported postweaning to NIMH, Bethesda, MD. Analysis of GAL-R1 −/− mice bred at The Jackson Laboratory and tested in our laboratory at NIMH has found no evidence of abnormal seizure activity (unpublished observations). Experiments began following an acclimation period of 8 weeks, when mice were aged 11 weeks or older. Subjects were from two separately bred cohorts. Cohort 1 consisted of 13 GAL-R1 −/−, 19 GAL-R1 +/−, and 18 +/+ male mice. Cohort 2 consisted of 13 GAL-R1 −/−, 13 GAL-R1 +/−, and 12 +/+ male mice. Factor analysis of behavioral scores from anxiety tests was conducted on data from both cohorts of GAL-R1 null mutant mice and their +/+ controls, combined with previously published data from two cohorts of galanin overexpressing GAL-tg mice and their +/+ controls (Steiner et al, 2001; Holmes et al, 2002b; Kinney et al, 2002), of both sexes. All four cohorts used for the factor analysis were on a C57BL/6J genetic background, bred and weaned at The Jackson Laboratory and tested on our anxiety behavior test battery using standardized procedures. 36 normal male C57BL/6J mice obtained from The Jackson Laboratory were used to test neuroendocrine responses following exposure to different tests for anxiety-like behavior. These mice were obtained at approximately 8–10 weeks of age and allowed to acclimate for 4 weeks prior to testing. All mice were housed 3–5 per cage with same-sex littermates, in a temperature- and humidity-controlled vivarium, under a 12-h light/dark cycle (lights on 0600 h). All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee, and followed the NIH guidelines ‘Using Animals in Intramural Research.’
Behavioral Testing
Anxiety-like behavior in GAL-R1 null mutant mice and +/+ littermate controls was assessed using a battery of tests as previously described (Holmes et al, 2002a,2002b). Testing was conducted in the following order, with a minimum of 1 week between tests: elevated plus-maze, light/dark exploration, emergence, open field. In each case, the apparatus was cleaned with a 10% ethanol solution and dried between subjects. To ensure experimenters remained blind to genotype, subjects were identified by barcode microchip scanner after the completion of each task. To minimize carryover effects of experimental manipulation on behavior in the anxiety-like test battery (Holmes and Rodgers, 1998,1999; Holmes et al, 2001b), the neurological, motor and sensory evaluation was conducted after the anxiety test battery. All testing was conducted during the light phase of the light/dark cycle (0600–1800 h).
Elevated Plus-Maze
The elevated plus-maze procedure was conducted as previously described (Holmes et al, 2002a,2002b). The apparatus (San Diego Instruments, San Diego, CA) comprised two open arms (30 × 5 cm2) and two closed arms (30 × 5 × 15 cm3) that extended from a common central platform (5 × 5 cm2). A small raised lip (0.5 cm) around the perimeter of the open arms prevented the mouse from falling. The apparatus was constructed from polypropylene and Plexiglas, with white floor and clear walls, and elevated to a height of 38 cm above floor level. Mice were individually placed on the center square, facing an open arm, and allowed to freely explore the apparatus under overhead fluorescent lighting (200 lx) for 5 min. Open and closed arm entries (all four paws in an arm) and time spent in the open arms were scored by a highly trained observer using behavioral scoring software (Hindsight, Scientific Programming Services, Wokingham, UK).
light/dark Exploration Test
The light/dark exploration test was conducted as previously described (Mathis et al, 1995; Holmes et al, 2002a,2002b). The apparatus consisted of a polypropylene cage (44 × 21 × 21 cm3) separated into two compartments by a partition, which had a small aperture (12 × 5 cm2) at the floor level. The larger compartment (28 cm long) was open-topped, transparent, and brightly illuminated by white light from a (40 W) desk lamp (1000 lx). The smaller compartment (14 cm long), was close topped and painted black. Mice were individually placed in the center of the light compartment, facing away from the partition, and allowed to freely explore the apparatus for 10 min. The number of light/dark transitions between the two compartments, and the total time spent in the dark compartment, were automatically recorded via photocells located at the intercompartmental aperture, connected to a data storage device (fabricated by Bruce Smith, George Dold, and co-workers, Research Services Branch, NIH, Bethesda, MD).
Emergence Test
The emergence test was a modified version of that previously described (Smith et al, 1998). The apparatus consisted of an opaque black Plexiglas cube (16 × 16 × 19 cm3) with a small aperture (6 × 4 cm2) on one side at the floor level. This ‘shelter’ was placed within an open field (40 × 40 × 35 cm3) evenly illuminated by white overhead fluorescent lighting (550 lx), with the exit facing out into the open field (parallel with one wall of the open field). Mice were individually placed inside the shelter with the exit closed for a 5 min habituation period. After the habituation period, the exit was opened and the mouse was allowed to leave the shelter and freely explore the open field for 5 min. The latency to emerge from the shelter, time out of the shelter, time spent engaged in risk assessment (scanning the open field with the head, but less than four paws out of the shelter), and shelter/open field transitions were recorded by a highly trained observer using behavioral scoring software (Hindsight, Scientific Programming Services, Wokingham, UK).
Open Field Test
Spontaneous exploratory activity and anxiety-like behavior were assessed in an automated open field, as previously described (Holmes et al, 2001a,2002a,2002b). To retain the novelty of the apparatus, following previous experience of an open field in the emergence test procedure, mice were tested in different open field chambers, housed in a different test room, from those used for the emergence test. The open field was a square arena (40 × 40 × 35 cm3) with clear Plexiglas walls and floor, evenly illuminated by white overhead fluorescent lighting (550 lx). Mice were individually placed in the center of the open field and left to explore freely for 30 min. Activity was measured by a computer-assisted Digiscan optical animal activity system (RXYZCM, Omnitech Electronics, Accuscan, Colombus, OH). Eight photocell beams were located on each of the two sides of the arena, at right angles to one another, forming a grid of 64 equally sized squares. To detect vertical movements, a third set of eight photocell beams were located above the square grid. The number of horizontal and vertical beam breaks was taken as measures of horizontal and vertical activity, respectively. Time spent in a central square (20 × 20 cm2) of the open field was automatically recorded as center time.
Sensory, Motor, and Neurological Assessment
GAL-R1 −/− and GAL-R1 +/− mice, and +/+ littermate controls were examined for general health, basic motoric abilities, neurological reflexes, empty cage behaviors, motor coordination, acoustic startle responses, and prepulse inhibition of acoustic startle responses, as described previously (Crawley and Paylor, 1997; Crawley, 2000; Holmes et al, 2001a,2002b). General health was measured via body weight, fur, coat, and whisker condition. The basic motoric abilities measured were: the maintenance of positional passivity when placed on a 45°-inclined Plexiglas sheet, forepaw reaching when slowly lowered to a solid surface; trunk curl when suspended by the tail; righting reflex when placed in the supine position. Neuromuscular strength and stamina were tested using the wire hang test (eg Caston et al, 1999; Gerlai et al, 2000). For this test, the mouse gripped onto ∼5 mm gauge round metal bars. The latency for the mouse to lose its grip and fall onto a foam pad below was timed with a stopwatch over a 60 s period. Neurological reflexes included the toe pinch response, and the responses of the cornea, pinna and vibrissae to approach with a cotton swab. Mice individually placed in an empty cage were observed for initial freezing, wild running, stereotypies, exploration, and grooming.
Motor coordination and balance were assayed with an accelerating rotarod (Ugo Basile, Stoelting, Wood Dale, IL) as previously described (Holmes et al, 2001a). Mice were placed on a slowly rotating drum, which gradually accelerated from 4 to 40 rpm over a 5 min period. The latency to fall onto a platform ∼8 cm below was timed using a stopwatch.
Sensitivity to painful stimuli was evaluated using the hot-plate and tail-flick tests. For the hot-plate test, mice were placed on a hot plate (ITTC Inc., Woodland Hills, CA), heated to 55°C (±0.3). The latency to make a hind paw lick was timed with a stopwatch. To prevent any tissue damage there was a maximum cutoff latency of 30 s. For the tail-flick test, mice were gently held in place with the tail lying along the groove of the tail-flick monitor (Columbus Instruments, Columbus, OH). An intense photobeam was directed at the tail and the latency to move the tail out of the path of the beam was timed with a stopwatch. The intensity of the photobeam had been adjusted such that a tail-flick latency of 3 s was obtained in control mice. To prevent any tissue damage there was a maximum cutoff latency of 10 s.
Acoustic startle and prepulse inhibition were measured in separate experiments using the SR-Lab System (San Diego Instruments, San Diego, CA) as described previously (Paylor and Crawley, 1997; Holmes et al, 2001a). Test sessions began by placing the mouse in the Plexiglas holding cylinder for a 5-min acclimation period. For acoustic startle, mice were presented with each of nine trial types across four discrete blocks for a total of 36 trials, over a 9-min test session. Trial types constituted the presentation of 70, 74, 78, 82, 86, 90, 100, 110, and 120 dB sound bursts. For prepulse inhibition of acoustic startle responses, mice were presented with each of seven trial types across six discrete blocks for a total of 42 trials, over a 10.5-min test session. One trial measured the response to no stimulus (ie baseline movement). One trial presented the startle stimulus alone (ie acoustic amplitude), which was a 40 ms 120 dB sound burst. The other five trials were acoustic prepulse+acoustic startle stimulus trials. Prepulse tones were 20 ms at 74, 78, 82, 86, and 90 dB, presented 100 ms before the startle stimulus.
For both acoustic startle and prepulse inhibition experiments, the order in which trial types were presented was randomized within each trial block. The interval between trials was 10–20 s. Startle amplitude was measured every 1 ms, over a 65 ms period beginning at the onset of the startle stimulus. The maximum startle amplitude over this sampling period was taken as the dependent variable. A background level of 70 dB white noise was maintained over the duration of the test sessions.
Factor Analysis of Behavior in Tests for Anxiety-Related Behaviors
Factor analysis was performed on behavioral scores of 279 mice from four separate cohorts of GAL-R1 null mutant and GAL-tg mice, and their respective +/+ littermate controls tested on our anxiety test battery, employing statistical methods previously described (Holmes and Rodgers, 1998; Wolfer et al, 1998; Fernandes et al, 1999). Factor pattern matrices were initially identified using the Kaiser criterion (ie eigenvalues ⩾1). To ensure that factors were independent from one another, a principal components solution with orthogonal rotation (varimax) of the factor matrices was used. Principal components factor analysis organizes within-subject data from multiple tests according to the strength of correlations between behavioral scores. Highly correlating behaviors are sorted into common factors, with the size of a given factor loading (0–1.0) denoting the strength of a behavior within the factor (Child, 1994).
Neuroendocrine Responses to Test Exposure
Plasma ACTH and corticosterone levels following a 10 min exposure to either the elevated plus-maze, light/dark exploration, or emergence test were measured in normal male C57BL/6J mice. Subjects left undisturbed in the home cage served as baseline controls. Following test exposure or, in the case of controls, removal from the home cage, mice were immediately sacrificed via rapid decapitation and trunk blood collected. In order to minimize variation because of diurnal changes in circulating neuroendocrine levels, all blood samples were taken between 1000 and 1300 h. Plasma was assayed for adrenocorticotropin (ACTH) and corticosterone by radioimmunoassay using methods described previously (Li et al, 1999).
Briefly, for the measurement of ACTH, 20 μl plasma samples or ACTH (1–39) standards (0.1–20 pg) were incubated with ACTH antiserum (IgG corp., Nashville, TN) (final dilution, 1 : 30 000) at 4°C overnight in 0.01 M PBS, pH 7.6, containing 1% BSA, 0.025 M EDTA, 0.5% normal rabbit serum, and 25 kallikrein-inactivating units (KIU)/ml aprotinin. 125I-ACTH (DIASORIN, Stillwater, MN) (2000 cpm/0.1 ml) was added and incubated for 24 h at 4°C in a total volume of 0.3 ml. After incubation with the second antibody (1 : 50 final dilution, goat anti-rabbit γ-globulin (Calbiochem, San Diego, CA) at 4°C for 24 h, 1.5 ml of cold PBS was added to the tubes, and they were then centrifuged at 15 000g and 4°C for 40 min. The radioactivity of the pellet was counted with a Micromedic 4/600 gamma counter. The sensitivity of the assay was 0.2 pg/tube, and the intra-assay and interassay variations were 4.2 and 14.6%, respectively.
For measurement of corticosterone, 3 μl of plasma or corticosterone standards (Sigma, St Louis, MO) (0.01–5 ng) were added into 0.5 ml of total volume assay buffer (0.1 M PBS, 0.1% gelatin, and 0.1% sodium azide) and incubated at 75°C for 20 min to denature corticosterone-binding proteins. After the tubes were cooled to room temperature, corticosterone antiserum (Costa Mesa, CA) (final dilution, 1 : 11 200) and [3H]corticosterone (at 7000 cpm) were added and incubated at 4°C overnight. The free and bound [3H]corticosterone were separated by incubating 0.2 ml of Carcoal suspension (Sigma, St Louis, MO) (62.5 mg Destran-T70 and 0.625% Norit A in assay buffer) for 20 min at 4°C, followed with centrifugation at 15 000g and 4°C for 20 min. The supernatant was decanted into scintillation vials containing 5 ml of scintillation fluid (Scint 30, Fisher) and counted in a Beckman scintillation counter. The sensitivity was 0.02 ng/tube, and the intra-assay and interassay variations were 4.5 and 11.9%, respectively.
STATISTICS
The effect of genotype on behavioral measures and the effect of test type on neuroendocrine response were analyzed using between-subject analysis of variance (ANOVA), and Newman–Keuls post hoc tests in the presence of significant ANOVA effects, using StatView (SAS Institute Inc., Cary, NC). Within-subject scores for open field activity over time were analyzed using repeated measures of ANOVA. Factor analysis was performed using Statistica (StatSoft Inc., Tulsa, OK).
RESULTS
Elevated Plus-Maze
Cohort 1
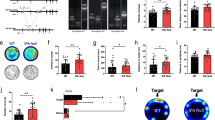
As shown in Figure 1a, b, GAL-R1 −/− spent significantly less time in the open arms (ANOVA, F(1,41)=4.30, p=0.02; post hoc tests, p<0.05 +/+ vs −/−, p>0.05 +/+ vs +/−) and made significantly fewer entries into the open arms (ANOVA F(1,41)=5.62, p=0.007; post hoc tests, p<0.05 +/+ vs −/−, p>0.05 +/+ vs +/−) than +/+ controls. There was no effect of genotype on total entries (F(1,41)=0.30, p=0.74) or closed entries (F(1,41)=0.16, p=0.16).
GAL-R1 −/− show greater anxiety-like behavior than +/+ and +/− littermates in the elevated plus-maze test, as demonstrated in two independent groups of mice (cohorts 1 and 2). GAL-R1 −/− showed less exploration of the open arms than +/+ controls (a, b). There were no genotype differences on control measures of locomotor activity in the elevated plus-maze (c, d) (cohort 1: n=18 +/+, 15 +/−, 11 −/−; cohort 2: n=11 +/+, 12 +/−, 11 −/−). *p<0.05 vs +/+.
Cohort 2
As shown in Figure 1a, b, GAL-R1 −/− spent less time in the open arms than +/+ controls (ANOVA, F(1,31)=3.61, p=0.04, post hoc tests, p<0.05 +/+ vs −/−, p>0.05 +/+ vs +/−). There was no significant genotype effect on percentage of open entries (F(1,31)=1.77, p=0.19), total entries (F(1,31)=0.65, p=0.53), or closed entries (F(1,31)=2.11, p=0.14).
Light/Dark Exploration Test
Cohort 1
As shown in Figure 2a, b, there was no effect of genotype on light/dark transitions (F(2,46)=2.13, p=0.13) or percentage of time in the dark compartment (F(2,46)=0.87, p=0.43).
GAL-R1 −/− were similar to +/+ and +/− littermates in the light/dark exploration test for anxiety-like behavior, as demonstrated in two independent cohorts. There were no genotype differences on light/dark transitions and percentage of time spent in the dark compartment (a, b) (cohort 1: n=17 +/+, 19 +/−, 13 −/−; cohort 2: n=12 +/+, 12 +/−, 13 −/−).
Cohort 2
As shown in Figure 2a, b, there was no effect of genotype on light/dark transitions (F(2,34)=2.28, p=0.12) or percentage time in the dark compartment (F(2,34)=2.19, p=0.13).
Emergence Test
Cohort 1
As shown in Figure 3b, GAL-R1 −/− made significantly fewer shelter/open field transitions than +/+ controls (ANOVA, F(2,42)=5.95, p=0.005; post hoc tests, p<0.05 +/+ vs −/−, p>0.05 +/+ vs +/−). As shown in Figure 3a, c, there was no significant effect of genotype on latency to exit the shelter (F(2,42)=1.74, p=0.19), percentage of time out of the shelter (F(2,42)=2.23, p=0.12), or percentage of risk assessment (F(2,42)=2.15, p=0.13; data not shown).
GAL-R1 −/− were similar to +/+ and +/− littermates in the emergence test for anxiety-like behavior, as demonstrated in two independent cohorts. GAL-R1 −/− from cohort 1 made fewer shelter/open field transitions than +/+ controls (b). There were no genotype differences on latency to exit the shelter (a), or time out of the shelter (c). *p<0.05 vs +/+ (cohort 1: n=17 +/+, 15 +/−, 13 −/−; cohort 2: n=11 +/+, 10 +/−, 11 −/−).
Cohort 2
As shown in Figure 3a–c, there was no significant effect of genotype on latency to exit the shelter (F(2,29)=0.36, p=0.70), shelter/open field transitions (F(2,29)=1.89, p=0.17), percentage of time out of the shelter (F(2,29)=0.94, p=0.40), or percentage of risk assessment (F(2,29)=0.72, p=0.50; data not shown).
Open Field Test
Cohort 1
As shown in Figure 4a–c, there was no significant effect of genotype on horizontal activity (F(2,43)=0.52, p=0.23), vertical activity (F(2,40)=0.31, p=0.73), or percentage of center time (F(2,43)=0.85, p=0.43). There was no significant genotype × time interaction on horizontal activity (F(5,15)=0.46, p=0.91). Generally, mice showed significant decreases in horizontal activity over the test session (F(5,200)=44.59, p<0.001).
GAL-R1 −/− were similar to +/+ and +/− littermates in the open field test for exploratory locomotor activity and anxiety-like behavior, as demonstrated in two independent cohorts. There were no genotype differences for horizontal locomotor activity (a), vertical activity (b), or percentage of center time (c) (cohort 1: n=16 +/+, 18 +/−, 12 −/−; cohort 2: n=11 +/+, 13 +/−, 11 −/−).
Cohort 2
As shown in Figure 4a–c, there was no significant effect of genotype on horizontal activity (F(2,32)=0.93, p=0.41), vertical activity (F(2,32)=1.26, p=0.30), or percentage of center time (F(2,32)=0.76, p=0.48). There was no significant genotype × time interaction for horizontal activity (F(10,160)=0.33, p=0.97). Generally, mice showed significant decreases in horizontal activity over the test session (F(5,160)=11.15, p<0.001).
Factor Analysis
Results are shown in Table 1. Factor 1, accounting for 25% of the variance, comprised high loadings for open field horizontal and vertical activity, and percentage of center time (negative) in the open field, and also a moderate loading for closed entries in the elevated plus-maze. Factor 2 (24% of the variance) comprised moderate to high loadings for light/dark transitions and percentage of time in the dark compartment (negative) in the light/dark exploration test, as well as a high loading for latency to out shelter (negative) and a moderate loading for shelter/open field transitions in the emergence test. Factor 3 (12% of the variance) had high loadings for percentage of open time and percentage of open entries in the elevated plus-maze test. Factor 4 (9% of the variance) comprised a moderate loading for shelter/open field transitions and a high loading for percentage of time out of the shelter in the emergence test. Lastly, Factor 5 (8% of the variance) comprised a single high factor for percentage of time in risk assessment in the emergence test.
ACTH and Corticosterone Responses to Test Exposure
In normal C57BL/6J mice, ANOVA found a significant effect of test type on plasma ACTH (F(3,32)=15.61, p<0.001) and corticosterone (F(3,32)=4.55, p<0.01). Newman–Keuls post hoc analysis showed that plasma ACTH and corticosterone responses were significantly elevated, as compared to home-cage controls, for all three tests. As shown in Figure 5a, exposure to the elevated plus-maze resulted in a significantly greater ACTH response, as compared to exposure to the light/dark test (p<0.01). Corticosterone increases after elevated plus-maze exposure were not significantly different than after exposure to the other two tests (Figure 5b).
Neuroendocrine responses to exposure to different tests for anxiety-like behavior in normal C57BL/6J mice. Significant increases in plasma levels of adrenocorticotropin and corticosterone were detected after 10 min exposure to the elevated plus-maze, light/dark exploration, and emergence tests, as compared to nonexposed controls (baseline). Plasma adrenocorticotropin was highest in mice exposed to the elevated plus-maze (n=9 baseline, n=8 light/dark exploration test, n=10 emergence test, n=9 elevated plus-maze test). **p<0.01; *p<0.05 vs baseline. p<0.01 vs light/dark exploration test.
Sensory, Motor, and Neurological Assessment
As shown in Table 2, GAL-R1 null mutant mice (cohort 1) were normal on measures of general health, as compared to +/+ controls, that is, body weight, fur condition, missing whiskers, and bald patches. Measures of basic motor ability, including positional passivity, trunk curl, forepaw reaching, righting reflex, and wire hang latency, were all normal in GAL-R1 −/−. The cornea, pinna, vibrissae, and toe pinch reflexes were intact in all genotypes. In an empty cage, GAL-R1 mutant mice and +/+ controls showed no initial freezing, wild running, or stereotypies, and genotypes showed similar levels of exploration and grooming. On the accelerating rotarod test, there was no significant effect of genotype on latency to fall (F(2,49)=0.27, p=0.76). Mice of all genotypes showed increased latencies on successive daily trials (F(2,98)=37.22, p<0.001). There was no interaction between genotype and trial on latency to fall (F(2,98)=1.50, p=0.21). There was no significant effect of genotype on latency to respond to an aversive stimulus in either the hot-plate (F(2,55)=0.06, p=0.94) or tail-flick (F(2,49)=0.38, p=0.69) assays. There was no significant effect of genotype on startle amplitude responses to acoustic startle stimuli ranging from 70 to 120 dB (F(2,49)=0.18, p=0.84). Generally, mice showed progressively higher startle amplitude at louder acoustic levels (F(8,392)=89.57, p<0.001) (only startle amplitudes to 74 and 120 dB stimuli are shown in Table 2). There was no interaction between genotype and startle level on acoustic startle amplitude (F(8,392)=1.15, p=0.31). There was a significant interaction between genotype and prepulse level on prepulse inhibition (F(4,196)=2.20, p=0.03), and a significant main effect of prepulse intensity (F(4,196)=77.89, p<0.001), but not genotype (F(2,49)=0.57, p=0.58). Post hoc analysis comparing genotypes at each individual prepulse intensity level found that there were no differences in prepulse inhibition at any prepulse intensity (74 dB prepulse inhibition of startle response is shown in Table 2). Results were centered in cohort 2 (data not shown).
DISCUSSION
Present findings show that mice lacking the gene for the galanin GAL-R1 receptor subtype (GAL-R1 −/−) exhibit evidence of heightened anxiety-like behavior that is preferentially manifest under more stressful test conditions. A battery of tests was used to assess anxiety-like behavior in GAL-R1 null mutant mice. In the elevated plus-maze test, GAL-R1 −/− displayed significantly less exploration of the aversive open arms than +/+ controls, consistent with heightened anxiety-like behavior. Importantly, control measures of locomotor activity in this test, that is, total and closed arm entries, were no different between GAL-R1 −/− and +/+. Moreover, on other separate measures of motor behavior, including motor coordination on the accelerating rotarod, and horizontal and vertical activity in the open field, GAL-R1 −/− were no different from +/+ controls. These data are consistent with a selective increase in anxiety-like behavior in GAL-R1 −/− in the elevated plus-maze test, unrelated to motor impairment.
In other measures of anxiety-like behavior, GAL-R1 −/− were similar to +/+ littermate controls. Thus, neither the frequency of light/dark transitions, nor the time spent in the dark compartment was affected by genotype in the light/dark exploration test. In the emergence test, another task based on a light/dark, approach/avoid conflict, GAL-R1 −/− again showed no clear anxiety-like phenotype. Although there was a decrease in shelter/open field transitions in GAL-R1 −/− relative to +/+ controls, the latency to exit the shelter and overall time spent out of the shelter was not different between genotypes. Finally, center time in the open field, which is a less rigorous measure of anxiety-related behavior, was not affected by genotype. These findings demonstrate that heightened anxiety-like behavior in GAL-R1 −/− is not generalized across multiple assays but, rather, is specific to the elevated plus-maze test.
We exclude the possibility that heightened anxiety-like behavior in the elevated plus-maze was a false positive finding, because a second, independent cohort of GAL-R1 −/− replicated the anxiety-like phenotype in this test. Moreover, supporting the specificity of this phenotype to the elevated plus-maze, there was again no evidence of abnormal anxiety-like behavior in the light/dark exploration, emergence, or open field tests in the second cohort. Further, false positives were excluded in measures of general health, body weight, neurological reflexes, and several sensory and motor abilities, which were all normal in GAL-R1 −/−. Therefore, our replication of a selective increase in anxiety-like behavior in the elevated plus-maze in GAL-R1 −/−, as compared to +/+, across two independent cohorts of mice, provides strong confirmation that the GAL-R1 receptor mutation induces a highly selective anxiogenic-like phenotype.
An anxiety-related phenotype in the two cohorts of GAL-R1 −/− mice was confirmed despite differences in the absolute behavioral scores between the cohorts. Generally, irrespective of genotype, cohort 2 displayed somewhat lesser anxiety-like behavior in the elevated plus-maze and light/dark explorations tests, and increased open field locomotor activity, relative to cohort 1. The reasons for the baseline differences between these two experiments, using batches of mice that were separately raised and tested, remain unknown. These data most likely reflect the natural variation in scores on anxiety-related behaviors across different cohorts of mice. This variation was present even though genetic background, breeding, weaning and housing conditions, experimental procedure, test order and behavioral analysis were carefully controlled (see also Crabbe et al, 1999; Holmes et al, 2002b). Such effects may also have modified the elevated plus-maze anxiety-like phenotype in GAL-R1 −/− mice, which appeared to be somewhat stronger on the first batch than the second.
Many reports demonstrate abnormal anxiety-like phenotypes in mutant mice that are consistent across multiple anxiety-related tasks, including 5-HT1A receptor, corticotrophin-related hormone CRH-R1 receptor, and GABAA γ2 subunit null mutants (for a review, see Holmes, 2001). Using the standardized anxiety-test battery in our laboratory, we have recently observed a highly consistent anxiety-like phenotype in serotonin transporter null mutant mice (Holmes et al, 2001c). However, there is growing evidence that ostensibly similar rodent behavioral tests may measure different forms of anxiety-like behavior (Belzung and LePape, 1994; File, 1995; Holmes and Rodgers, 1998; Ramos and Mormede, 1998; van Gaalen and Steckler, 2000). In an analysis of behavior from the elevated plus-maze, light/dark exploration, open field, square maze, and mirror chamber, Turri et al (2001) have recently found that some genetic quantitative trait loci (QTL) are common to anxiety-like behaviors on multiple tests, while others are specific to a given task. This suggests that different tests for anxiety-like behavior are affected by distinct, but partly overlapping, genes. In support of this argument, previous studies have also found that targeted gene mutations can produce anxiety-like phenotypes in some tests, but not others. For example, two groups found that corticotrophin-related hormone CRH-R2 receptor null mutant mice show increased anxiety-like behavior in the elevated plus-maze, but not the light/dark exploration or open field tests (Bale et al, 2000; Kishimoto et al, 2000), while a third group found an anxiety-like phenotype in CRH-R2 −/− on the open field, but not elevated plus-maze (Coste et al, 2000).
Factor analysis has been employed as a statistical approach to examine commonality and dissimilarity between different behavioral test paradigms, including anxiety-related tests (Royce et al, 1975; Zola-Morgan et al, 1994; Griebel et al, 1996; Holmes and Rodgers, 1998; Ramos et al, 1998; Fernandes et al, 1999; Aguilar et al, 2002). A large sample of data from GAL-R1 null mutant, galanin overexpressing GAL-tg mice, and their respective controls assessed on our anxiety-test battery under consistent local conditions were subject to principal components factor analysis. Data were resolved in a five-factor solution. The main finding was that measures of anxiety-like behavior in the elevated plus-maze (open time, open entries) loaded separately (on factor 3) from four other factors varyingly comprised of behaviors in the light/dark exploration, emergence and open field tests. This pattern is consistent with previous reports in rats (File, 1995; Ramos et al, 1998; Fernandes et al, 1999; Aguilar et al, 2002) and supports the notion that behavioral responses on the elevated plus-maze are qualitatively distinct from those on the other three tests. Measures from the light/dark exploration (time spent in the dark, light/dark transitions) and emergence (latency to exit shelter, shelter/open field transitions) tests coloaded on factor 2. The composition of this factor suggests that the light/dark exploration and emergence tests measure similar forms of anxiety-like behavior. However, the fact that additional emergence test behaviors (factor 4: time out of shelter, shelter/open field transitions; factor 5: risk assessment) loaded separately from the combined light/dark exploration/emergence test factor, indicates that there are also differences between the two tests. Finally, factor 1 comprised strong positive loadings for horizontal and vertical activity in the open field and closed entries in the elevated plus-maze, plus a high negative loading for open field center time. The coloading of elevated plus-maze closed entries with measures of open field activity provides support for the interpretation of closed entries as an index of exploratory locomotor activity in the elevated plus-maze that can be differentiated from anxiety-related responses in the test (Holmes and Rodgers, 1998; Fernandes et al, 1999).
When factor analysis was conducted on scores from nonmutant wild-type and +/+ control mice only, a similar factor solution was obtained, suggesting that genetic differences did not influence the pattern of differences between tests for anxiety-like behavior. However the present factor analysis was conducted on mice that were all on a C57BL/6J genetic background and it would be of interest to determine whether similar results would be found with mice from other, genetically distinct mouse strains.
Exposure to tests for anxiety-like behavior, including the elevated plus-maze, is known to increase levels of plasma stress hormones (Pellow et al, 1985; File et al, 1994; Rodgers et al, 1999). To test the hypothesis that qualitative differences between tests for anxiety-like behavior were paralleled by differential stress hormone responses to test exposure, plasma ACTH and corticosterone levels were measured in normal C57BL/6J mice immediately following exposure to the elevated plus-maze, light/dark exploration, or emergence tests. Our data show a significantly greater ACTH response after elevated plus-maze exposure than exposure to the light/dark exploration test in normal C57BL/6J mice. Emergence test responses were intermediate. Plasma corticosterone was similarly elevated in C57BL/6J mice exposed to either of the three tests. The absence of differential corticosterone levels following test exposure may reflect the slower time course of the corticosterone response to stress. Thus, because corticosterone is released into the circulation from the adrenal cortex following stimulation by ACTH, the corticosterone response may not have reached peak levels in plasma 10 min after test exposure (Spinedi et al, 1992; Groenink et al, 1994). These data provide preliminary evidence to suggest that anxiety-like behavior on the elevated plus-maze may not only be qualitatively distinct from anxiety-like behaviors on other tests in our battery, but may also reflect a response to a more stressful test situation.
Taken together with the literature on the effects of galanin on the behavioral, neuroendocrine, and sympathetic responses to stressors, described in the Introduction, the present findings support the hypothesis that galanin exerts anxiolytic actions preferentially under stressful conditions (Moller et al, 1999; Holmes et al, 2002b; Khoshbouei et al, 2002a,2002b). Our data suggest that the GAL-R1 subtype contributes to the anxiolytic actions of endogenous galanin. In normal mice, galanin released in response to stressful conditions would reduce the stress-induced anxiety-like response. This anxiolytic-like effect of endogenous galanin would be lost in the GAL-R1 null mutants. In less stressful conditions, endogenous galanin would not be released and no anxiety-like phenotype would be evident in GAL-R1 null mutant mice. This model is in agreement with the frequency-dependent nature of galanin release. In contrast to classical neurotransmitters, which are released at low neuronal firing rates, neuropeptides such as galanin are released only during high neuronal firing, as might be expected under high stress provocation (Bartfai et al, 1988; Consolo et al, 1994; Hökfelt et al, 2000). Interestingly in this regard, GAL-R1 +/− mice demonstrated normal anxiety-like behaviors on all tests. While autoradiographic analysis of GAL-R1 receptor binding in GAL-R1 mutant mice has not yet been conducted, GAL-R1 +/− mice would be expected to show a 50% reduction in normal GAL-R1 receptor expression. As such, normal anxiety-like behavior in these mice suggests that a threshold level of functional GAL-R1 receptor inactivation is required to produce an anxiety-like phenotype. It will be important to test whether both GAL-R1 −/− and GAL-R1 +/− mice are compromised under conditions of more severe or chronic stress.
Anxiolytic effects of the GAL-R1 receptor in response to stress may be mediated via effects on the ascending noradrenergic system, which is implicated in the pathophysiology of anxiety disorders (Bremner et al, 1996; Ressler and Nemeroff, 2000). Galanin immunoreactivity coexists with tyrosine hydroxylase immunoreactivity in the majority of noradrenergic cell bodies projecting to forebrain regions underlying emotion (Melander et al, 1986; Holets et al, 1988; Hökfelt et al, 1998b; Perez et al, 2001). Galanin reduces the firing rate and hyperpolarizes noradrenergic neurons of the locus coeruleus, and inhibits the release of norepinephrine from terminals located in the cortex (Seutin et al, 1989; Tsuda et al, 1989; Sevcik et al, 1993; Pieribone et al, 1995; Xu et al, 1998a,1998b). A recent study supports a role for the GAL-R1 receptor in these effects. Ma et al (2001) found that the galanin receptor agonist, AR-M1896, which has 500-fold selectivity for the GAL-R2 vs the GAL-R1 receptor, failed to mimic the inhibitory effects of galanin on locus coeruleus cell firing and hyperpolarization in vitro. This led the authors to suggest that the GAL-R1 receptor mediates galanin's inhibitory effects on noradrenergic neuronal activation, whereas GAL-R2 receptors, probably located presynaptically, may regulate norepinephrine release in forebrain areas. Differential pre- vs postsynaptic activation of GAL-R2 vs GAL-R1 receptors could underlie previously reported region-specific effects of galanin ligands on anxiety-like behavior in rats (Bing et al, 1993; Moller et al, 1999; Khoshbouei et al, 2002a,2002b).
In GAL-R1 −/−, the absence of the normal inhibitory effects of galanin on locus coeruleus neurons may remove an important inhibition of norepinephrine activation during stress, leading to increased anxiety-like behavior. To test this hypothesis further, it will be important to study electrophysiological characteristics of locus coeruleus neurons and norepinephrine release in GAL R1 −/− under stressful conditions (Kehr et al, 2001). In addition, interactions between galanin and serotonin function have been reported (Fuxe et al, 1998; Xu et al, 1998a; Razani et al, 2001; Kehr et al, 2002). Future studies on functional interactions between these systems in the mediation of stress and anxiety will also be valuable to further our understanding of the role of galanin in the pathophysiology of emotional disorders and their treatment.
In summary, GAL-R1 receptor null mutant showed heightened anxiety-like behavior in two cohorts of mice in the elevated plus-maze. Scores were normal on the light/dark exploration, emergence, and open field tests. Factor analysis of behavioral scores derived from these tests demonstrated that anxiety-like behavior on the elevated plus-maze was qualitatively distinct from behaviors in the other tests. Exposure to the elevated plus-maze produced a significantly greater increase in plasma ACTH than exposure to the light/dark exploration test in normal C57BL/6J. Taken together, these findings suggest that the absence of the GAL-R1 receptor leads to increased anxiety-like behavior during relatively stressful test situations, consistent with an anxiolytic role for galanin acting at the GAL-R1 receptor subtype following strong emotional arousal. These findings have implications for the development of novel therapeutic targets for stress- and anxiety-related disorders. A GAL-R1 receptor agonist could exert anxiolytic effects under conditions of extreme or persistent negative affect, as in anxiety disorders and depression, while remaining silent in everyday situations where normal responses to mild stressors are desirable.
References
Aguilar R, Gil L, Flint J, Gray JA, Dawson GR, Driscoll P et al (2002). Learned fear, emotional reactivity and fear of heights: a factor analytic map from a large F(2) intercross of Roman rat strains. Brain Res Bull 21: 17–26.
Austin MC, Cottingham SL, Paul SM, Crawley JN (1990). Tyrosine hydroxylase and galanin mRNA levels in locus coeruleus neurons are increased following reserpine administration. Synapse 6: 351–357.
Bale TL, Contarino A, Smith GW, Chan R, Gold LH, Sawchenko PE et al (2000). Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet 24: 410–414.
Bartfai T, Iverfeldt K, Fisone G, Serfozo P (1988). Regulation of the release of coexisting neurotransmitters. Annu Rev Pharmacol Toxicol 28: 285–310.
Belzung C, Le Pape G (1994). Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav 56: 623–628.
Bing O, Moller C, Engel JA, Soderpalm B, Heilig M (1993). Anxiolytic-like action of centrally administered galanin. Neurosci Lett 164: 17–20.
Branchek TA, Smith KE, Gerald I, Walker MW (2000). Galanin receptor subtypes. Trends Pharmacol Sci 21: 109–117.
Bremner JD, Krystal JH, Southwick SM, Charney DS (1996). Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse 23: 28–38.
Carey DG, Iismaa TP, Ho KY, Rajkovic IA, Kelly J, Kraegen EW et al (1993). Potent effects of human galanin in man: growth hormone secretion and vagal blockade. J Clin Endocrinol Metab 77: 90–93.
Caston J, Devulder B, Jouen F, Lalonde R, Delhaye-Bouchaud N, Mariani J (1999). Role of an enriched environment on the restoration of behavioral deficits in Lurcher mutant mice. Dev Psychobiol 35: 291–303.
Ceresini G, Sgoifo A, Freddi M, Musso E, Parmigiani S, Del Rio G et al (1998). Effects of galanin and the galanin receptor antagonist galantide on plasma catecholamine levels during a psychosocial stress stimulus in rats. Neuroendocrinology 67: 67–72.
Child D (1994). The Essentials of Factor Analysis, 2nd edn. Cassell: London.
Consolo S, Baldi G, Russi G, Civenni G, Bartfai T, Vezzani A (1994). Impulse flow dependency of galanin release in vivo in the rat ventral hippocampus. Proc Natl Acad Sci USA 91: 8047–88051.
Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH et al (2000). Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet 24: 403–409.
Crabbe JC, Wahlsten D, Dudek BC (1999). Genetics of mouse behavior: interactions with laboratory environment. Science 284: 1670–1672.
Crawley JN (2000). What's Wrong with My Mouse? Behavioral Phenotyping of Transgenic and Knockout Mice. Wiley-Liss: New York.
Crawley JN, Paylor RA (1997). Proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav 31: 197–211.
degli Uberti EC, Ambrosio MR, Bondanelli M, Trasforini G, Margutti A, Valentini A et al (1995a). Human galanin reduces plasma norepinephrine levels in man. J Clin Endocrinol Metab 80: 1894–1898.
degli Uberti EC, Ambrosio MR, Bondanelli M, Trasforini G, Valentini A, Rossi R et al (1995b). Effect of human galanin on the response of circulating catecholamines to hypoglycemia in man. Eur J Endocrinol 133: 723–728.
Fernandes C, Gonzalez MI, Wilson CA, File SE (1999). Factor analysis shows that female rat behaviour is characterized primarily by activity, male rats are driven by sex and anxiety. Pharmacol Biochem Behav 64: 731–738.
File SE (1995). Animal models of different anxiety states. Adv Biochem Psychopharmacol 48: 93–113.
File SE, Zangrossi H, Sanders FL, Mabbutt PS (1994). Raised corticosterone after exposure to the elevated plus-maze. Psychopharmacology 113: 543–546.
Fuxe K, Jansson A, Diaz-Cabiale Z, Andersson A, Tinner B, Finnman UB et al (1998). Galanin modulates 5-hydroxytryptamine functions. Focus on galanin and galanin fragment/5-hydroxytryptamine1A receptor interactions in the brain. Ann NY Acad Sci 863: 274–290.
Gerlai R, Thibodeaux H, Palmer JT, van Lookeren Campagne M, Van Bruggen N (2000). Transient focal cerebral ischemia induces sensorimotor deficits in mice. Behav Brain Res 108: 63–71.
Griebel G, Blanchard DC, Blanchard RJ (1996). Evidence that the behaviors in the Mouse Defense Test Battery relate to different emotional states: a factor analytic study. Physiol Behav 60: 1255–1260.
Groenink L, van der Gugten J, Zethof T, van der Heyden J, Olivier B (1994). Stress-induced hyperthermia in mice: hormonal correlates. Physiol Behav 56: 747–749.
Habert-Ortoli E, Amiranoff B, Loquet I, Laburthe M, Mayaux JF (1994). Molecular cloning of a functional human galanin receptor. Proc Natl Acad Sci USA 91: 9780–9783.
Hökfelt T, Bartfai T, Crawley JN (1998a). Galanin: basic research discoveries and therapeutic implications. Ann NY Acad Sci, Vol. 863. New York Academy of Sciences: New York.
Hökfelt T, Broberger C, Diez M, Xu ZQ, Shi T, Kopp J et al (1999). Galanin and NPY, two peptides with multiple putative roles in the nervous system. Horm Metab Res 31: 330–334.
Hökfelt T, Broberger C, Xu ZQ, Sergeyev V, Ubink R, Diez M (2000). Neuropeptides—an overview. Neuropharmacology 39: 1337–1356.
Hökfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X (1998b). Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann NY Acad Sci 863: 252–263.
Holets VR, Hökfelt T, Rokaeus A, Terenius L, Goldstein M (1988). Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience 24: 893–906.
Holmes A (2001). Transgenic and gene knockout approaches to the study of anxiety-like behavior in mice. Neurosci Biobehav Rev 25: 261–273.
Holmes A, Hollon TR, Liu Z, Sibley DR, Dreiling J, Gleason TC et al (2001a). Dopamine D5 receptor null mutant mice show attenuated behavioral responses to a dopamine agonist. Behav Neurosci 115: 1129–1144.
Holmes A, Iles JP, Mayell SJ, Rodgers RJ (2001b). Prior test experience compromises the anxiolytic efficacy of chlordiazepoxide in the mouse light/dark exploration test. Behav Brain Res 122: 159–167.
Holmes A, Rodgers RJ (1998). Responses of Swiss–Webster mice to repeated plus-maze experience: further evidence for a qualitative shift in emotional state? Pharmacol Biochem Behav 60: 473–488.
Holmes A, Rodgers RJ (1999). Influence of spatial and temporal manipulations on the anxiolytic efficacy of chlordiazepoxide in mice previously exposed to the elevated plus-maze. Neurosci Biobehav Rev 23: 971–980.
Holmes A, Wrenn CC, Harris AP, Thayer K, Crawley JN (2002a). Behavioral profiles of inbred strains on novel, olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav 1: 55–69.
Holmes A, Yang RJ, Crawley JN (2002b). Evaluation of an anxiety-related phenotype in galanin overexpressing transgenic mice. J Mol Neurosci 18: 151–165.
Holmes A, Yang RJ, Murphy DL, Crawley JN (2001c). Abnormal emotional behaviors and age-related obesity in 5-HT transporter mice. Soc Neurosci Abstr 27: 985.7.
Holmes PV, Blanchard DC, Blanchard RJ, Brady LS, Crawley JN (1995). Chronic social stress increases levels of preprogalanin mRNA in the rat locus coeruleus. Pharmacol Biochem Behav 50: 655–660.
Holmes PV, Crawley JN (1995). Coexisting neurotransmission in central noradrenergic neurons. In: Bloom FE, Kupfer DJ (eds). Psychopharmacology: The Fourth Generation of Progress. Raven Press: New York. pp 347–353.
Hooi SC, Maiter DM, Martin JB, Koenig JI (1990). Galaninergic mechanisms are involved in the regulation of corticotropin and thyrotropin secretion in the rat. Endocrinology 127: 2281–2289.
Iismaa TP, Shine J (1999). Galanin and galanin receptors. Results Probl Cell Differ 26: 257–291.
Jacoby AS, Holmes FE, Hort YJ, Shine J, Iismaa TP (2002a). Phenotypic analysis of GAL-R1 knockout mice reveals a role for GALR1 galanin receptor in modulating seizure activity but not nerve regeneration. Lett Pept Sci 8: 139–146.
Jacoby AS, Hort YJ, Constantinescu G, Shine J, Iismaa TP (2002b). Critical role for GAL-R1 galanin receptor in galanin regulation of neuroendocrine function and seizure activity. Brain Res Mol Brain Res 107: 195–200.
Kehr J, Yoshitake T, Wang F, Razani H, Gimenez-Llort L, Jansson A et al (2002). Galanin is a potent in vivo modulator of mesencephalic serotonergic neurotransmission. Neuropsychopharmacology 27: 341–356.
Kehr J, Yoshitake T, Wang FH, Wynick D, Holmberg K, Lendahl U et al (2001). Microdialysis in freely moving mice: determination of acetylcholine, serotonin and noradrenaline release in galanin transgenic mice. J Neurosci Methods 109: 71–80.
Khoshbouei H, Cecchi M, Dove S, Javors M, Morilak DA (2002a). Behavioral reactivity to stress. Amplification of stress-induced noradrenergic activation elicits a galanin-mediated anxiolytic effect in central amygdala. Pharmacol Biochem Behav 71: 407–417.
Khoshbouei H, Cecchi M, Morilak DA (2002b). Modulatory effects of galanin in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuropsychopharmacology 27: 25–34.
Kinney JW, Starosta G, Holmes A, Wrenn CC, Yang RJ, Harris AP et al (2002). Deficits in trace cued fear conditioning in galanin-treated rats and galanin-overexpressing transgenic mice. Learn Memory 9: 178–190.
Kishimoto T, Radulovic J, Radulovic M, Lin CR, Schrick C, Hooshmand F et al (2000). Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet 24: 415–419.
Li Q, Wichems C, Heils A, Van de Kar LD, Lesch KP, Murphy DL (1999). Reduction of 5-hydroxytryptamine (5-HT)(1A)-mediated temperature and neuroendocrine responses and 5-HT(1A) binding sites in 5-HT transporter knockout mice. J Pharmacol Exp Ther 291: 999–1007.
Ma X, Tong YG, Schmidt R, Brown W, Payza K, Hodzic L et al (2001). Effects of galanin receptor agonists on locus coeruleus neurons. Brain Res 919: 169–174.
Makino S, Asaba K, Nishiyama M, Hashimoto K (1999). Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology 70: 160–167.
Malendowicz LK, Nussdorfer GG, Nowak KW, Mazzocchi G (1994). The possible involvement of galanin in the modulation of the function of rat pituitary–adrenocortical axis under basal and stressful conditions. Endocr Res 20: 307–317.
Mathis C, Paul SM, Crawley JN (1995). Characterization of benzodiazepine-sensitive behaviors in the A/J and C57BL/6J inbred strains of mice. Behav Genet 24: 171–180.
Mazarati A, Langel U, Bartfai T (2001). Galanin: an endogenous anticonvulsant? Neuroscientist 7: 506–517.
Melander T, Hökfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A et al (1986). Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci 6: 3640–3654.
Merchenthaler I, Lopez FJ, Negro-Vilar A (1993). Anatomy and physiology of central galanin-containing pathways. Prog Neurobiol 40: 711–769.
Moller C, Sommer W, Thorsell A, Heilig M (1999). Anxiogenic-like action of galanin after intra-amygdala administration in the rat. Neuropsychopharmacology 21: 507–512.
O'Donnell D, Ahmad S, Wahlestedt C, Walker P (1999). Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol 409: 469–481.
O'Neal HA, Van Hoomissen JD, Holmes PV, Dishman RK (2001). Prepro-galanin messenger RNA levels are increased in rat locus coeruleus after treadmill exercise training. Neurosci Lett 299: 69–72.
Palkovits M (2000). Stress-induced expression of co-localized neuropeptides in hypothalamic and amygdaloid neurons. Eur J Pharmacol 405: 161–166.
Parker EM, Izzarelli DG, Nowak HP, Mahle CD, Iben LG, Wang J et al (1995). Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res Mol Brain Res 34: 179–189.
Paylor R, Crawley JN (1997). Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology 132: 169–180.
Pellow S, Chopin P, File SE, Briley M (1985). Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167.
Perez SE, Wynick D, Steiner RA, Mufson EJ (2001). Distribution of galaninergic immunoreactivity in the brain of the mouse. J Comp Neurol 434: 158–185.
Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hökfelt T (1995). Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience 64: 861–874.
Ramos A, Mellerin Y, Mormede P, Chaouloff F (1998). A genetic and multifactorial analysis of anxiety-related behaviours in Lewis and SHR intercrosses. Behav Brain Res 96: 195–205.
Ramos A, Mormede P (1998). Stress and emotionality: a multidimensional and genetic approach. Neurosci Biobehav Rev 22: 33–57.
Razani H, Diaz-Cabiale Z, Misane I, Wang FH, Fuxe K, Ogren SO (2001). Prolonged effects of intraventricular galanin on a 5-hydroxytryptamine(1A) receptor mediated function in the rat. Neurosci Lett 299: 145–149.
Ressler KJ, Nemeroff CB (2000). Role of serotonergic and noradrenergic systems in the pathophysiology of depression and anxiety disorders. Depress Anxiety 12: 2–19.
Rodgers RJ, Haller J, Holmes A, Halasz J, Walton T, Brain PF (1999). Corticosterone response to the plus-maze: high correlation with risk assessment in rats and mice. Physiol Behav 68: 47–53.
Royce JR, Holmes TM, Poley W (1975). Behavior genetic analysis of mouse emotionality. III. The diallel analysis. Behav Genet 5: 351–372.
Saar K, Mazarati AM, Mahlapuu R, Hallnemo G, Soomets U, Kilk K et al (2002). Anticonvulsant activity of a nonpeptide galanin receptor agonist. Proc Natl Acad Sci USA 99: 7136–7141.
Scott MK, Ross TM, Lee DH, Wang HY, Shank RP, Wild KD et al (2000). 2,3-Dihydro-dithiin and -dithiepine-1,1,4,4-tetroxides: small molecule non-peptide antagonists of the human galanin hGAL-1 receptor. Bioorg Med Chem 8: 1383–1391.
Seutin V, Verbanck P, Massotte L, Dresse A (1989). Galanin decreases the activity of locus coeruleus neurons in vitro. Eur J Pharmacol 164: 373–376.
Sevcik J, Finta EP, Illes P (1993). Galanin receptors inhibit the spontaneous firing of locus coeruleus neurones and interact with mu-opioid receptors. Eur J Pharmacol 230: 223–230.
Skofitsch G, Jacobowitz DM (1986). Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides 7: 609–613.
Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold L et al (1998). Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron 20: 1093–1102.
Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK (1999). Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci 113: 558–566.
Spinedi E, Suescun MO, Hadid R, Daneva T, Gaillard RC (1992). Effects of gonadectomy and sex hormone therapy on the endotoxin-stimulated hypothalamo-pituitary–adrenal axis: evidence for a neuroendocrine-immunological sexual dimorphism. Endocrinology 131: 2430–2436.
Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A et al (2001). Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc Natl Acad Sci USA 98: 4184–4189.
Sweerts BW, Jarrott B, Lawrence AJ (1999). Expression of preprogalanin mRNA following acute and chronic restraint stress in brains of normotensive and hypertensive rats. Brain Res Mol Brain Res 69: 113–123.
Sweerts BW, Jarrott B, Lawrence AJ (2000). Acute and chronic restraint stress: effects on [125I]-galanin binding in normotensive and hypertensive rat brain. Brain Res 873: 318–329.
Tsuda K, Yokoo H, Goldstein M (1989). Neuropeptide Y and galanin in norepinephrine release in hypothalamic slices. Hypertension 14: 81–86.
Turri MG, Datta SR, DeFries J, Henderson ND, Flint J (2001). QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol 11: 725–734.
van Gaalen MM, Steckler T (2000). Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res 115: 95–106.
Wang S, He C, Maguire MT, Clemmons AL, Burrier RE, Guzzi MF et al (1997). Genomic organization and functional characterization of the mouse GalR1 galanin receptor. FEBS Lett 411: 225–230.
Waters SM, Krause JE (2000). Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95: 265–271.
Wolfer DP, Stagljar-Bozicevic M, Errington ML, Lipp HP (1998). Spatial memory and learning in transgenic mice: fact or artifact? News Physiol Sci 13: 118–123.
Wrenn CC, Crawley JN (2001). Pharmacological evidence supporting a role for galanin in cognition and affect. Prog Neuropsychopharmacol Biol Psychiatry 25: 283–299.
Xu ZQ, Shi TJ, Hökfelt T (1998b). Galanin/GMAP- and NPY-like immunoreactivities in locus coeruleus and noradrenergic nerve terminals in the hippocampal formation and cortex with notes on the galanin-R1 and -R2 receptors. J Comp Neurol 392: 227–251.
Xu ZQ, Zhang X, Pieribone VA, Grillner S, Hökfelt T (1998a). Galanin-5-hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience 87: 79–94.
Zola-Morgan S, Squire LR, Ramus SJ (1994). Severity of memory impairment in monkeys as a function of locus and extent of damage within the medial temporal lobe memory system. Hippocampus 4: 483–495.
Acknowledgements
This research was supported by the NIMH Intramural Research Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holmes, A., Kinney, J., Wrenn, C. et al. Galanin GAL-R1 Receptor Null Mutant Mice Display Increased Anxiety-Like Behavior Specific to the Elevated Plus-Maze. Neuropsychopharmacol 28, 1031–1044 (2003). https://doi.org/10.1038/sj.npp.1300164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300164
Keywords
This article is cited by
-
Ucp4 Knockdown of Cerebellar Purkinje Cells Induces Bradykinesia
Molecular Neurobiology (2024)
-
Comprehensive behavioral analyses of mice with a glycine receptor alpha 4 deficiency
Molecular Brain (2023)
-
Conditional deletion of Neurexin-2 alters neuronal network activity in hippocampal circuitries and leads to spontaneous seizures
Translational Psychiatry (2023)
-
The Pivotal Role of Neuropeptide Crosstalk from Ventromedial-PACAP to Dorsomedial-Galanin in the Appetite Regulation in the Mouse Hypothalamus
Molecular Neurobiology (2023)
-
Human ARHGEF9 intellectual disability syndrome is phenocopied by a mutation that disrupts collybistin binding to the GABAA receptor α2 subunit
Molecular Psychiatry (2022)