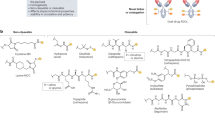
Abstract
Platinum-based chemotherapeutics exhibit excellent antitumor properties. However, these drugs cause severe side effects including toxicity, drug resistance, and lack of tumor selectivity. Tumor-targeted drug delivery has demonstrated great potential to overcome these drawbacks. Herein, we aimed to design radioactive bisphosphonate-functionalized platinum (195mPt-BP) complexes to confirm preferential accumulation of these Pt-based drugs in metabolically active bone. In vitro NMR studies revealed that release of Pt from Pt BP complexes increased with decreasing pH. Upon systemic administration to mice, Pt-BP exhibited a 4.5-fold higher affinity to bone compared to platinum complexes lacking the bone-seeking bisphosphonate moiety. These Pt-BP complexes formed less Pt-DNA adducts compared to bisphosphonate-free platinum complexes, indicating that in vivo release of Pt from Pt-BP complexes proceeded relatively slow. Subsequently, radioactive 195mPt-BP complexes were synthesized using 195mPt(NO3)2(en) as precursor and injected intravenously into mice. Specific accumulation of 195mPt-BP was observed at skeletal sites with high metabolic activity using micro-SPECT/CT imaging. Furthermore, laser ablation-ICP-MS imaging of proximal tibia sections confirmed that 195mPt BP co-localized with calcium in the trabeculae of mice tibia.
Similar content being viewed by others
Introduction
Most types of tumors, i.e. breast, prostate, lung, kidney, and thyroid, metastasize to bone since its physiological environment facilitates the formation and growth of cancer cells1,2. These metastases affect healthy bone, which then become the primary cause of mortality3. Distant metastases are the leading cause of death for both breast and prostate cancer patients, with 65–75% and 90% of these patients developing bone metastases in advanced stages, respectively4,5. Bone metastases are often associated with accelerated bone resorption leading to complications such as skeletal-related events (SREs), bone pain or hypercalcemia6,7. Unfortunately, current treatments for bone metastases are limited. Bisphosphonates (BP) and denosumab are most commonly used for palliative treatment to prevent or limit SREs8. Although such treatments inhibit osteoclast activity and prevent the progression of metastases, they do not kill cancer cells effectively and do not improve the quality of life of patients substantially9. Consequently, the development of effective therapies to treat bone metastases remains a major clinical challenge.
Cancer cells modulate the bone microenvironment supporting tumor growth and accelerating tumor progression10. The elimination of cancer cells from bone metastases is crucial for treatment efficacy, which requires precise and efficient delivery of antitumor therapeutics to bone metastases. Clinically, bone-targeting radiopharmaceuticals such as 153Sm-ethylenediaminetetramethylenephosphonate and 89Sr-chloride are widely applied β-emitters for bone palliation11. Currently, 223Ra-dichloride is approved as a bone-seeking calcium-mimetic radiopharmaceutical for treatment of metastatic castration-resistant prostate cancer patients by emitting α-particles12. Moreover, clinical trials have been performed to combine 223Ra treatment with additional chemotherapy to enhance therapeutic efficacy13,14. Generally, the combination of chemotherapy with radionuclide therapy may give rise to a radiosensitizing effect where a chemotherapeutic component enhances tumor cell radiation sensitivity15,16.
Among various types of radiosensitizers, platinum-based (Pt) drugs belong to a class of radiosensitizers that influence the nature or repair of DNA damage17. Moreover, Pt-based drugs have also been investigated as radiotherapeutics by incorporation of radioactive platinum isotopes18,19,20. The incorporation of radioactive Pt atoms further enhances the therapeutic properties of Pt-based drugs21,22. Notably, radioisotope 195mPt with a half-life of 4 days, is considered to be the most suitable Pt isotope for radionuclide therapy and medical imaging, since it is a prolific Auger electron emitter and emits a 99 keV gamma for SPECT (single-photon emission computed tomography) imaging, respectively23,24. Therefore, targeted delivery of Pt-based chemotherapeutics comprising 195mPt could potentially increase the therapeutic efficacy of Pt-based drugs. Such targeted theranostic approach for treatment of bone metastases has the potential for precision medicine to “see and treat bone metastases”25.
Among the limited number of studies that evaluated the therapeutic properties of 195mPt, Bodnar et al. demonstrated that inhibition of the growth of Ehrlich solid carcinoma by 195mPt-cisplatin was 30% more effective compared to non-radioactive cisplatin23. Nevertheless, Pt based drugs are known to induce systemic toxicity, while the lack of tumor specificity restricts their application for long-term treatment26,27. Consequently, tumor-targeted delivery of Pt has strong potential to overcome the drawbacks of the currently applied Pt-based drugs28. Margiotta et al. formulated various anticancer Pt-bisphosphonate complexes aimed at targeted delivery of Pt to bone29,30,31, since BPs accumulate in bone of high metabolic activity32. This feature might potentially enable targeted delivery of Pt-based chemotherapeutics to bone metastases in view of the high metabolic activity of bone metastases33,34. However, the bisphosphonate ligands of Pt-BP compounds are presented in an entirely different manner to the mineral phase of bone as compared to free bisphosphonate distribution. Therefore, we aimed to study the biodistribution of Pt-BP compounds in metabolically active bone using advanced elemental mapping and micro-SPECT imaging techniques.
To this end, we design novel radioactive Pt-BP (195mPt-BP) complexes to enable targeted delivery of 195mPt to bone of high metabolic activity. First, we confirm the in vivo bone-seeking and Pt-DNA adduct-forming capacity of Pt-BP complexes 24 h post-administration using high-resolution inductively coupled plasma-mass spectrometry (ICP-MS). Second, we monitor the long-term stability and bone-seeking capacity of 195mPt-BP over a 7-day period using preclinical micro-SPECT/CT imaging in mice. Finally, we show the spatiotemporal distribution of 195mPt BP within the mice tibia using laser ablation ICP-MS imaging35.
Results
Pt-BP favors bone-specific delivery of Pt with reduced Pt-DNA adduct formation
We comparatively evaluated the bone-seeking properties of Pt-BP and its bisphosphonate-free precursor Pt(NO3)2(en) by quantitatively analyzing Pt biodistribution using Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) in different tissues (e.g. tibia, femur, humerus, spine, blood, heart, lung, kidney, liver, and spleen) as shown in Fig. 1A. To this end, we intravenously injected sterile saline solutions containing Pt-BP or Pt(NO3)2(en) with identical Pt concentrations into the tail vein of mice, which were euthanized 24 h after injection. The dissected tissues were weighed and digested in 65% nitric acid for subsequent ICP-MS analysis of Pt content. Figure 1B shows the Pt concentrations in different tissues 24 h after injection, normalized for specific tissue weight. Pt-BP clearly showed bone-seeking properties as evidenced by higher amounts of Pt accumulation (~3 ng Pt/mg tissue) in hard tissue (bone) compared to BP-free control Pt(NO3)2(en) complex (~1 ng Pt/mg tissue).
Biodistribution profile of Pt-BP and Pt(NO3)2(en) in vivo. (A) Schematic representation of Pt biodistribution study in C57Bl/6N mice. Pt-BP or Pt(NO3)2(en) compounds were administered intravenously in mice (2.5 mM Pt concentration) followed by sacrifice after 24 h. Hard tissues (tibia, femur, humerus, spine), soft tissues (heart, lungs, kidney, liver, spleen) and blood were collected. Collected tissues were subjected to nitric acid digestion and genomic DNA extraction for Pt quantification using ICP-MS and high-resolution ICP-MS, respectively. (B) Pt concentration in different tissues was normalized for specific tissue weight 24 h after injection. Data from 5 mice per group are represented as ng Pt/mg tissue ± SD. (C) Percentage of Pt uptake in different tissues 24 h after injection. Data from 5 mice per group are represented as % I.D./g ± SD of specific tissue type. (D) Percentage of Pt-DNA adduct formation relative to total Pt uptake in specific tissue as quantified by High Resolution-ICP-MS. Data from 4–5 mice per group are presented. **P < 0.01; ***P < 0.001; ****P < 0.0001 as determined by two-way ANOVA with a Bonferroni (multiple comparisons) post-hoc test.
The specificity of the two different Pt-species towards bone tissue relative to other soft tissues is depicted in Fig. 1C as the percentage of injected dose per gram of tissue (%ID/g). Higher uptake of the Pt-BP was observed in hard tissue (12.18 ± 0.56%ID/g) compared to excretory organs such as the kidney (5.70 ± 0.15%ID/g), liver (2.34 ± 0.17%ID/g) and spleen (1.22 ± 0.12%ID/g). On the other hand, Pt(NO3)2(en) showed higher uptake in kidney (3.38 ± 0.28%ID/g) compared to hard tissue (2.69 ± 0.26%ID/g), liver (1.54 ± 0.33%ID/g) and spleen (0.38 ± 0.04%ID/g). Overall, Pt-BP exhibit a 4.5-fold higher affinity for bone compared to Pt(NO3)2(en) (Fig. 1C).
The efficacy of Pt-based drugs for cancer treatment relates to the formation of Pt-DNA adducts resulting in DNA damage, which hinder mitotic processes and halt cell division. Drawbacks of the formation of Pt-DNA adducts include the high rate of DNA damage in non-target cells or healthy tissue36. We determined the extent of Pt-DNA adduct formation by extracting genomic DNA from different tissues and quantifying the amount of Pt using high-resolution ICP-MS. Figure 1D shows the relative Pt uptake within specific tissues leading to Pt-DNA adduct formation 24 h after injection. Pt-BP formed a low amount of Pt-DNA adducts in all tissues (<0.5%) except for the kidney (2.8%) and spleen (1.4%), which confirms the relatively low extent of DNA damage in non-targeted tissue. In contrast, the bisphosphonate-free Pt complexes showed a much higher extent of Pt-DNA adduct formation, especially in the kidneys (4.8%) and spleen (9.8%).
195mPt-BP accumulates specifically in metabolically active bone
Radioactive 195mPt-BP was synthesized using 195mPt(NO3)2(en) as the precursor for 195mPt. To compare the biodistribution of 195mPt-BP with the precursor 195mPt(NO3)2(en), a dose of 11.2 ± 0.4 MBq 195mPt was administered intravenously via the tail vein in C57BL/6N mice. Although the administered Pt dose was below the Pt toxicity dose of 6 mg/kg for mice37, injection of 195mPt(NO3)2(en) evoked a significant body weight loss within the first 3 days post injection (p.i.), leading to euthanization on reaching a humane end point. On the other hand, no behavioral or body changes were observed for 195mPt-BP treated mice.
Micro-SPECT/CT images were acquired 1 h – 7 days post intravenous administration of 195mPt-BP (Fig. 2A). The micro-SPECT/CT scans clearly demonstrated effective targeting of 195mPt-BP to growth plates in long bones, whereas 195mPt(NO3)2(en) showed specific accumulation in soft tissues (Fig. 2B). Generally, growth plates are metabolically active regions in bones of young mice since they are responsible for longitudinal bone growth38. After excision of tissues, we quantified the uptake of radioactive 195mPt-BP complexes by gamma counting (Fig. 3A). Bone-specific uptake of 195mPt-BP was highest in the femur (3.1 ± 0.37%ID/g) followed by the tibia (2.86 ± 0.46%ID/g) and humerus (2.53 ± 0.33%ID/g), whereas 19mPt(NO3)2(en) showed limited uptake in these bones (1.4 ± 0.15%ID/g). 195mPt uptake was highest in soft tissue for 195mPt(NO3)2(en) with significantly higher uptake in kidneys (3.95 ± 0.24%ID/g), liver (3.22 ± 0.28%ID/g), spleen (2.76 ± 0.67%ID/g), and lungs (1.08 ± 0.16%ID/g) compared to 195mPt-BP. Conversely, 195mPt-BP showed relatively low uptake in soft tissues (<0.11%ID/g), except for kidneys (0.43 ± 0.16%ID/g).
Biodistribution profile of 195mPt-BP and 195mPt(NO3)2(en) in vivo. Representative whole-body micro-SPECT/CT images of biodistribution of 195mPt-BP (A) and 195mPt(NO3)2(en) (B) in nude mice 24 hours after systemic administration. Rectangles show specific biodistribution of these compounds in long bones (femur-tibia) 24 h, 72 h and 168 h after systemic administration.
Quantification of 195mPt-BP and 195mPt(NO3)2(en) biodistribution in vivo. (A) Percentage of injected dose (%ID/g) of 195mPt-BP (after 7 days) and 195mPt(NO3)2(en) (after 3 days) in mice determined using gamma counting. (B) Percentage of injected dose (%ID/g) of 195mPt-BP and 195mPt(NO3)2(en) in mice as quantified from the micro-SPECT/CT images in soft and hard tissues. (C) Percentage of injected dose (%ID/g) of 195mPt-BP and 195mPt(NO3)2(en) in mice as quantified from the micro-SPECT/CT images in the hard tissue region of interest (ROI) with the location of the edge of the ROI contour representing 75% of maximum intensity. (D) 195mPt hard-to-soft tissue uptake ratio excluding bladder uptake at 1 h. Data from 4–5 mice per group are presented. **P < 0.01; ****P < 0.0001 as determined by two-way ANOVA with a Bonferroni (multiple comparisons) post-hoc test. For the ratios, paired t-test was used to determine the differences among the two groups where **P < 0.01 was considered as significantly different.
Next, we analyzed the micro-SPECT/CT images quantitatively to determine the uptake of radioactive 195mPt-BP complexes. 195mPt-BP showed rapid and strong uptake in bone (1.8 ± 0.25%I.D/g) at 1 h p.i., which remained constant (1.0 ± 0.12%I.D/g) from 24 h until day 7. 195mPt-BP uptake was significantly lower in soft tissue than in hard tissue (0.93 ± 0.27%I.D/g at 1 h and 0.18 ± 0.03%I.D/g at 24 h). In contrast, 195mPt(NO3)2(en) showed equal uptake in both hard (1.5 ± 0.1%I.D/g) and soft tissue (1.43 ± 0.21%I.D/g) at 1 h p.i., and the biodistribution remained unchanged until day 3 as shown in Fig. 3B. Subsequently, the quantification of the hot spots in bone represents a twofold increased uptake of 195mPt for 195mPt-BP compared to 195mPt(NO3)2(en) for all time points (Fig. 3C). The hard-to-soft tissue uptake ratio of 195mPt-BP increased periodically from 3.3 at 1 h, 6 at 24 h and 6.7 after 72 h. On the other hand, the hard-to-soft tissue uptake ratio of 195mPt(NO3)2(en) remained almost constant at 1 at 1 h, 1.2 at 24 h and 1.2 at 72 h (Fig. 3D).
Spatial co-localization of Pt with Ca in bone
The spatial distribution of Pt as determined using Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) is depicted in Fig. 4. The calcium and phosphorus distribution (Supplement Fig. S1) clearly conformed to the structure of trabecular bone as observed in the dark-colored features in the microscopic image (Fig. 4A). The overlay of Ca and Pt distinctly differentiates co-localization of Pt with Ca (yellow) from Pt surrounding the trabecular structure (green). 195mPt-BP showed predominant Pt uptake along the trabecular bone and inner region of the cortical bone, as reflected by an increased density of Pt co-localization with calcium. Conversely, 195mPt(NO3)2(en) showed the highest Pt density in the soft tissue surrounding the cortical bone.
Spatial distribution of Pt in metabolically active bone. (A) Representative elemental mapping of platinum (Pt) in the proximal tibia of mice upon systemic administration of 195mPt-BP and 195mPt(NO3)2en. The bottom pictures shown an overlay of calcium (red) and platinum (green) mapping where co-localization of platinum and calcium is indicated in yellow. (B) Percentage of platinum co-localized with calcium in 195mPt-BP and 195mPt(NO3)2(en) treated mice. ****P < 0.0001, two-tailed student’s test. (C) Total amount of Pt co-localized with Ca (in hard tissue, Pt ≈ Ca) and Pt not co-localized with Ca (in soft tissue, Pt ≠ Ca). Data are presented from 10 sections from three tibia per group. ****P < 0.0001 as determined by two-way ANOVA with a Bonferroni (multiple comparisons) post-hoc test.
To quantitatively examine the co-localization of Pt with Ca, we calculated the percentage of Pt co-localized with calcium. This calculation (Fig. 4B) shows a co-localization of Pt with calcium of 73.5% for 195mPt-BP and only 37.3% for 195mPt(NO3)2(en). Relative to the extent of co-localization of Pt with Ca as observed for 195mPt-BP, co-localization of 195mPt(NO3)2(en) was only 27% (Fig. 4C). The total amount of Pt not co-localized with Ca refers to Pt in soft tissue of the proximal tibia where 10% and 20% of Pt was detected in soft tissue for 195mPt(NO3)2(en) and 195mPt-BP, respectively. 195mPt-BP showed almost a fourfold increased accumulation of Pt in bone compared to 195mPt(NO3)2(en) as shown in Fig. 4C. These results confirm again that 195mPt-BP binds specifically to the bone and effectively penetrates the trabecular structure of mouse tibiae.
Discussion
Recent advances in imaging technologies allow early detection of malignant tissues directly in bone marrow39,40 or detection of indirect tumor activity related to bone remodeling41,42. Such early assessment of developing bone metastases would enable patient-specific bone-targeted treatment. However, treatment options for patients with bone metastases are scarce and palliative rather than curative4,5. Consequently, the design of novel theranostic compounds which detect and treat bone metastases in cancer patients would overcome this unmet need.
Our present work focuses on the design of radioactive and bone-seeking chemotherapeutics based on 195mPt. To this end, we synthesized a Pt-BP compound comprising two 195mPt moieties and a bone-seeking bisphosphonate group to target the compound specifically to metabolically active bone. This synthesis of Pt-BP was modified compared to previously reported synthesis strategies to facilitate introduction of radioactive 195mPt atoms by using Pt(NO3)2(en) as Pt precursor29,31. 195mPt(NO3)2(en) was obtained from Na2[195mPtCl4], which is the Pt precursor used for synthesis of radioactive cisplatin, thereby allowing for future up-scaling and clinical translation of 195mPt-BP23.
The release of therapeutically active Pt species from Pt-BP is vital to impart the desired chemotherapeutic effects on cancer cells. Previous in vitro studies on Pt-BP dissociation upon release from solid hydroxyapatite nanoparticles surface confirmed that Pt-BP does not simply split into BP and Pt31. Pt-BP most likely releases one Pt moiety in the form of PtCl2(en) or related species, whereas the other Pt is sequestered with the BP molecule due to the coordination with the free amino group as present in Pt-BP. This observation is further supported by similar results obtained upon releasing Pt-BP from silica xerogels29.
To understand Pt release behavior in vivo, we investigated Pt-BP dissociation in near-physiological (pH 7.4) and acidic conditions (pH 5.25) (Supplement Fig. S2). Pt-BP underwent hydrolysis into a mononuclear Pt-n-BP derivative at both pH values, characterized by faster release of Pt at pH 5.25 than at pH 7.4. At physiological pH, Pt-BP forms new species with asymmetric bisphosphonate groups, which results most likely from displacement of one coordinated oxygen atom of the bridging bisphosphonate ligand by either a chloride (120 mM concentration) or bisphosphonate-bound amino group43. Besides, a signal belonging to an as yet unknown species appeared at 15.32 ppm at pH 5.25. As expected, the formation of unsymmetrical species having the amino group coordinated to the Pt atom is not favored at acidic pH, since this moiety is protonated and hence not available for coordination to the metal.
To investigate the bone-seeking ability of Pt-BP, the bisphosphonate-free compound Pt(NO3)2(en) was selected as control, since this compound was also used as precursor for the synthesis of Pt-BP. The primary reason to select Pt(en)(NO3)2 as control was that, as demonstrated by NMR on the cold Pt-BP complex, 195mPt-BP releases 195mPt(en)(OH2)2]2+, that undergoes anation reactions in physiological-like conditions. The same fate occurs also for 195mPt(en)(NO3)2. In order to understand if the 195mPt-species released by hydrolysis of 195mPt-BP were distributed in tissues different from bone, we used the Pt-species released. Moreover, since 195mPt(en)(NO3)2 was also the precursor used by the supplier (NRG) of radioactive 195mPt in the synthesis of 195mPt-BP, our choice allowed to reduce costs and time for the synthesis of radioactive 195mPt-BP compounds. Generally, we hypothesized that the Pt(NO3)2(en) control compound lacked bone-seeking properties due to the absence of a mineral-binding moiety. Our in vivo biodistribution studies showed that Pt-BP compounds delivered Pt specifically to the bone. Although Pt uptake was specifically observed in hard tissues for Pt-BP compounds, this Pt did not form many Pt-DNA adducts after 24 h. These results corroborate the slow release of Pt from Pt-BP compounds observed in vitro at physiological pH. In contrast, Pt(NO3)2(en) showed increased Pt-DNA adduct formation in kidneys and the spleen, which are well-known off-target organs for Pt-based drugs44. On the contrary, the passive process of Pt-DNA adduct formation upon slow release of Pt from Pt-BP might be beneficial by diminishing undesired off-target effects.
The biodistribution of radioactive 195mPt-BP and 195mPt(NO3)2(en) compounds revealed a pattern similar to our previous observations using cold Pt-based therapeutics. The extended follow-up period of 7 days confirmed specific accumulation of 195mPt-BP within bone only. In contrast, 195mPt(NO3)2(en) specifically accumulated in kidney, liver, and spleen, thereby affecting the excretory organs and resulting in weight losses of more than 20%, which was set as humane end point. Previously, several studies have shown that cisplatin-treated mice (maximum tolerated dose of 6 mg/Kg) undergo rapid weight loss (~10%) followed by recovery within a week37. However, 195mPt(NO3)2(en) administration resulted in more pronounced off-target effects, most likely caused by the additional radiotherapeutic properties of 195mPt. The long retention time of 195mPt in soft tissues as observed for 195mPt(NO3)2(en) might increase radiation-induced damage caused by Auger electrons and gamma irradiation. These side-effects were not observed for 195mPt-BP due to a combination of high bone-targeting efficacy and rapid excretion from soft tissues. Aalbersberg et al. recently demonstrated the feasibility of applying 195mPt-based micro-SPECT imaging in small animals24. Herein, we show noninvasive detection of 195mPt in bones exhibiting high metabolic activity after systemic administration of 195mPt-BP. The trabecular tissue near the marrow cavity is the most metabolically active region within bones, the activity of which declines at a constant rate with aging45. Micro-SPECT/CT imaging with 195mPt-BP showed specific accumulation of 195mPt in the trabecular part of the tibia, femur, and humerus of mice. Although whole-body radioactivity in 195mPt-BP treated mice was just 2%ID on day 7 (Supplement Fig. S3), the half-life of 195mPt (t1/2 = 4 days)23,24 enabled quantitative, high resolution SPECT imaging. We further demonstrated 195mPt-BP specificity towards skeletal sites by an increasing hard-to-soft tissue contrast from 1 h until day 3. Moreover, LA-ICP-MS imaging confirmed Pt co-localization with calcium within the trabecular region of the tibia of mice. In contrast, LA-ICP-MS imaging of the Pt distribution in 195mPt(NO3)2(en)-treated mice showed preferential uptake in soft tissues (bone marrow and blood vessels) surrounding the trabecular bone. Thus, LA-ICP-MS imaging indicates that 195mPt(NO3)2(en) uptake in the skeletal system is mediated by a passive mechanism via blood perfusion within the vasculature of the skeletal system.
Based on our preclinical results, 195mPt-BP offers several advantages as a theranostic agent for treatment of bone metastases. First, BP moieties effectively target 195mPt to bone sites with high metabolic activity. This opens up new opportunities for bone tumor-target delivery of Pt-BP compounds, since bone metastases are typically characterized by very high metabolic activity. Second, off-target effects caused by 195mPt are decreased by this bone-specific targeting, rapid excretion within 24 h, and the slow release of Pt from Pt-BP. Third, 195mPt-BP compounds remain stable for at least 24 h after reaching the targeted site, thereby improving the imaging quality for qualitative and quantitative diagnosis. Indeed, our results show slow release of Pt from Pt-BP in vivo upon attachment to hydroxyapatite mineral, which is in agreement with the in vitro stability of Pt-BP as observed previously using NMR. However, the main goal of systemic delivery of 195mPt-BP is to deliver this 195mPt (a rich source of Auger electrons) specifically to bone metastases. Considering the vigorous bone remodeling and increased bone metabolic activity in bone metastatic lesions, we assume that release of 195mPt at such sites will be accelerated due to enhanced bone remodeling involving osteoclastic resorption at reduced pH. Generally, Pt-BP compounds dissociate at acidic pH, which means that release of Pt can be accelerated upon tumor-induced acidification. This hypothesis should be investigated in future studies in tumor-bearing mice. Nevertheless, further investigations are required for the translation of 195mPt-BP towards clinical application.
The main goal of the study was successfully achieved, i.e. synthesis of radioactive 195mPt-BP compounds which accumulated preferentially in metabolically active bone. Nevertheless, several shortcomings of the current study should be highlighted. First, the 195mPt precursor 195mPt(NO3)2(en) was used as the control compound since other radioactive 195mPt derivatives of platinum-based drugs were not available. Second, additional therapeutic efficacy beyond the cytostatic effect of Pt may arise from Auger electrons and gamma rays emitted by 195mPt, which we will investigate in a preclinical bone metastasis model in tumor-bearing mice by comparing therapeutic effects of radioactive and non-radioactive Pt-BP compounds46. Finally, Pt toxicity is a major concern for clinical applications of Pt-based drugs47. Nevertheless, we did not observe any Pt-based toxicity in 195mPt-BP-treated mice over a period of 7 days. However, further long-term investigation is required to confirm adverse effect on bone biomarkers. Moreover, we addressed potential toxicity of Pt released from Pt-BP compounds in a zebrafish model, since this model provides a 500-fold higher sensitivity for early assessment of toxic effects caused by Pt-based chemotherapeutics48,49. As such, the zebrafish model is a highly valuable model for initial ototoxicity screening. Visual inspection of kidneys based on size and shape revealed apparent shrinkage for cisplatin controls but not for Pt-BP. In addition, no visible phenotypic effects of Pt-BP were observed in zebrafish embryos up to a concentration of 100 µM (Supplement Fig. S4). Pt-BP revealed a dose-dependent ototoxic effect on zebrafish embryos 48 h post treatment, as confirmed by staining for and quantification of lateral line neuromast hair cells (Supplement Fig. S5).
In summary, we described the synthesis and biodistribution of a bone-seeking 195mPt-BP compound for active targeting of 195mPt to bone sites with high metabolic activity. It should be stressed that radioactive 195mPt-BP compounds with bone-seeking properties have not been reported before. Their synthesis is highly complex from a logistical perspective since access to radioactive 195mPt of high specific activity is very limited worldwide. In addition, biodistribution of radioactive or inactive Pt-BP compounds has never been confirmed in vivo. Our results show that for the first time using micro-SPECT imaging that radioactive 195mPt-BP compounds accumulate in metabolically active bone. Finally, this accumulation was confirmed for the first time in hard tissues using elemental mapping by means of Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). We envision that 195mPt-BP as a bone-seeking theranostic agent could meet the current clinical need to “see and treat” bone metastases in cancer patients and reduce off-target side effects of Pt-based drugs.
Methods
Study design
The objective of the study was to evaluate Pt-BP as potential therapeutic (Pt) or theranostic agent (195mPt) to selectively deliver Pt or 195mPt to metabolic active region of bone. All experiments were performed with five animals per group to reach statistical significance. Mice were randomized into different groups before administration of Pt/195mPt compounds. Blinding was applied in housing. In addition, biotechnicians responsible for Pt compounds administration and monitoring of animal welfare was blinded. Image acquisition in vivo and ex vivo was performed in a non-blinded manner. Data analysis was also performed in a non-blinded manner.
Materials
Na2SO4 and Ba(OH)2 were purchased from Sigma-Aldrich. 2-amino-1-hydroxyethane-1,1-diyl-bisphosphonic acid (AHBP-H4) was prepared following procedures reported previously29. Milli-Q water was used to dissolve the compounds. All other reagents were purchased from Sigma-Aldrich and used without further purification.
Synthesis and characterization of Pt-BP complex
Pt-BP complexes with ethylenediamine(en) have been chosen as lead compounds based on previous extensive investigation43,50. We modified our previously reported synthesis of dinuclear bis<ethylenediamineplatinum(II) > -2-amino-1-hydroxyethane-1,1-diyl-bisphosphonate complex (Pt-BP) by using Pt(NO3)2(en) as Pt precursor instead of Pt(OSO3)(OH2)(en)29,30,50,51,52. This Pt(NO3)2(en) facilitates the synthesis of radioactive Pt-BP complexes using 195mPt radioisotopes for future applications as a theranostic agent. Synthetic procedures are provided in the supplementary material.
Characterization via elemental analysis, Electrospray Ionisation-Mass Spectrometry (ESI-MS), and spectroscopic features (Supplement Figs. S6 and 7) shows consistency of the Pt-BP product with the one obtained using Pt(OSO3)(OH2)(en) as a Pt precursor29,30. The 1H-NMR spectrum of Pt-BP (Supplement Fig. S7) shows two broad singlets at 5.93 and 5.43 ppm assigned to the aminic protons of coordinated ethylenediamine29. The acidic conditions decelerated the exchange of the aminic protons with the deuterium of the solvent, allowing detection of the aminic protons in aqueous solution. The doublet centered at 3.34 ppm is assigned to the protons of the methylene group of the bisphosphonate. The 31P-NMR spectrum (Supplement Fig. S7B) of the Pt-BP in D2O shows a singlet with unresolved Pt satellites positioned at 37.60 ppm. This singlet can be assigned to the two phosphorus atoms of the phosphonic groups, which are magnetically equivalent and shifted at lower field (Δδ = 21 ppm) with respect to the free 2-amino-1-hydroxyethane-1,1-diyl-bisphosphonic acid (AHBP) ligand at the same pH29.
Preparation of 195mPt(NO3)2(en)
The 195mPt was produced as previously reported24. The specific activity of 195mPt(NO3)2(en) was 48,5 MBq/mg Pt. The radionuclide purity of 195mPt(NO3)2(en) as received from NRG (Petten, The Netherlands) is reported in Supplement Table S1.
Pt-BP pH stability assessed by NMR
31P NMR spectra were recorded on a Bruker Avance III 700 MHz instrument29. Standard pulse sequences were used for 31P{1H} (121.5 MHz) spectra. Chemical shifts (31P) were referenced to external H3PO4 (85% w/w; 0 ppm). A Crison Micro-pH meter Model 2002, equipped with Crison microcombination electrodes (5- and 3-mm diameter) and calibrated with Crison standard buffer solution at pH 4.01, 7.02, and 10.00, was used for pH measurements. The pH readings from the pH meter for D2O solutions are indicated as pD values and are uncorrected for the effect of deuterium on glass electrodes. The stability of Pt-BP in buffered solutions at 37 °C was assessed by 31P NMR spectroscopy as previously reported53. Pt-BP (∼4 mg) was dissolved in 0.8 mL of D2O containing (i) 50 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) buffer (pD = 7.4) and 120 mM NaCl or (ii) 50 mM 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pD = 5.25) and 120 mM NaCl. The resulting two solutions were transferred into NMR tubes and maintained at 37 °C. 31P NMR spectra were recorded over a period of 15 days. The relative concentrations of the individual species in solution were deduced from integration of the 31P signals.
Preparation of radioactive Pt-BP complex
The synthesis procedure was followed as described for the cold platinum-bisphosphonate complex provided in the supplementary material. The 195mPt(NO3)2(en) solution was received from NRG (Petten, the Netherlands). The pH of 195mPt(NO3)2(en) solution was neutralized to pH 7 using 1M NaOH and reconstituted in sterile saline solution. Characterization via elemental analysis and Electrospray Ionisation-Mass Spectrometry (ESI-MS) was not possible due to insufficient amount of residual 195mPt-BP. The amount of 195mPt (and thus 195mPt-BP) was limited due to limited supply by NRG (Petten, the Netherlands).
In vivo studies
All in vivo work was conducted in accordance with ISO standards and the principles set forth by the Revised Dutch Act on Animal Experimentation. The in vivo experiments were approved by the institutional Animal Welfare Committee of the Radboud University Medical Center (Radboudumc), Nijmegen, the Netherlands. For both the experiment, 10 male C57Bl/6N mice (Charles River), with an average weight of ~25 g and an age of approximately 6–8 weeks were housed in filter-topped cages (5 mice per cage) under non-sterile standard conditions provided with standard animal food and water ad libitum. The mice were allowed to adapt to laboratory conditions for 1 week before experimental use. The humane end point were defined as follows, body weight gain of> 20% within 10 days, no water or food intake, weight loss of >15% within 1–2 days or> 20% of normal body weight of that animal, serious circulatory or respiratory problems, behavioural changes (hyperactivity, passive, auto-mutilation), and morbidities such as impaired mobility or lethargy52.
In vivo biodistribution of cold Pt-species and quantification of Pt accumulation
The Pt-BP and Pt(NO3)2(en) complexes were administered intravenously in the tail vein of C57BL/6N mice (n = 5 per each platinum complex). Sterile saline solution (0.9% NaCl) was used to dissolve platinum complexes. The concentration of injected Pt-BP or Pt(NO3)2(en) solutions was 2.5 mM platinum, calculated based on the maximum tolerated dose for cisplatin of 6 mg per kg body weight of the mice37. The mice were euthanized with CO2 24 h after injection, after which blood (approximately 600 mg per mouse), liver, spleen, kidneys, heart, lungs, and bones (femur, humerus, tibia, and spine) were harvested. Approximately half of the tissues were prepared for inductively coupled plasma-mass spectrometry (ICP-MS) analysis by digestion in 65% (v/v) nitric acid at 75 °C for approximately three days until the tissues were digested completely. Each tissue was cut into three samples and the samples were weighed prior to digestion in nitric acid. The digested solutions were diluted with up to 6 ml of Milli-Q water to obtain 2% (v/v) nitric acid in order to measure platinum concentrations by ICP-MS (X series I, Thermo Electron Corporation). The detection limit for determining platinum concentration with ICP-MS was 1 ppb. The standard solutions were prepared from 1000 mg·1−1 platinum ICP standard Certipur (1.70341.0100, Merck) ranging from 1 ppb to 2500 ppb. The measured isotopes for platinum were 194Pt, 195Pt, 196Pt, and 198Pt. Furthermore, scandium (45Sc) was added as an internal standard, which was prepared with 1000 mg·1−1 scandium standard Certipur (1.19513.0100, Merck) to correct for matrix effects and long-term fluctuations of the measurement signal. The platinum concentrations in the various tissues were calculated relative to 1 mg of the specific tissue and represented as ng Pt/mgtissue. Three replicates of Pt-BP and Pt(NO3)2(en) solutions with volumes and platinum concentrations identical to the solutions injected to the mice (200 µl and 2.5 mM platinum, respectively) were also analyzed for their platinum content using ICP-MS as control. The percentage of injected dose (%ID) of Pt-BP and Pt(NO3)2(en) in each mouse tissue 24 h after injection was calculated by dividing the total amount of platinum per tissue through the total amount of platinum detected in the control solutions52.
Pt-DNA adducts quantification using High Resolution ICP-MS
The remaining half of the tissues from all mice were dissected and weighed for DNA extraction. DNA was isolated using DNeasy Blood & Tissue Kit (Qiagen, USA) for soft tissue and ChargeSwitch® gDNA Plant Kit (Thermofisher, USA) for bone samples. The DNA was digested with DNase I Solution (Thermofisher, USA) and filtered using 0.2 µm acrodisc GHP before analysis for Pt content using high resolution-ICP-MS (Element2, Thermo Finnigan). The detection limit for determining the platinum concentration was 2 ng/l. The standard solutions were prepared from 1000 mg·1−1 platinum ICP standard Certipur (1.70341.0100, Merck) ranging from 5 ppt to 10 ppb. The selected isotopes for platinum were 194Pt and 195Pt. Furthermore, thallium (Tl) was added as an internal standard and determined at a molecular mass of 205 Da, which was prepared using a 1000 mg·1−1 thallium standard (CGTL-1, Inorganic Ventures) to correct for matrix effects and long-term fluctuations of the analytical signal. The Pt-DNA adduct concentration is represented as percentage of Pt involved in adduct formation to the total amount of Pt accumulated in the specific tissue52.
In vivo biodistribution of radioactive Pt-species and micro-SPECT quantification of 195mPt biodistribution
The 195mPt-BP and 195mPt(NO3)2(en) complexes were reconstituted in sterile saline solution (0.9% NaCl) and administered intravenously in the tail vein of C57BL/6N mice (n = 5 per each platinum complex). All mice received an intravenous injection with a dose of 11.2 ± 0.4 MBq 195mPt. Immediately after injection and 1, 3, and 7 days after injection, images were acquired with U-SPECT-II/CT(MILabs) as reported previously54,55. Mice were scanned under general anesthesia (isoflurane/O2) for 15 to 60 minutes using the 1.0-mm diameter pinhole mouse high sensitivity collimator tube, followed by a CT scan (spatial resolution 160 mm, 65 kV, 615 mA) for anatomical reference. Scans were reconstructed with MILabs reconstruction software using an ordered-subset expectation maximization algorithm, with a voxel size of 0.4 mm. SPECT/CT scans were analyzed and maximum intensity projections were created using the Inveon Research Workplace software (IRW, version 4.1). A 3D volume of interest was drawn using CT threshold to differentiate soft tissue from skeletal tissue and uptake was quantified as the percentage injected dose per gram (%ID/g), assuming a tissue density of 1 g/cm3. The hot spot in the skeletal tissue region of interest (ROI) was chosen with the location of the edge of the ROI contour representing 75% of maximum intensity. The mice from 195mPt(NO3)2(en) group were euthanized with CO2 after 3 days due to excessive loss of body weight, whereas mice from treated with 195mPt-BP were sacrificed at the end of experiments. After euthanizing the animals, blood (approximately 600 mg per mouse), liver, spleen, kidneys, heart, lungs, and bones (femur, humerus, tibia, and spine) were harvested and analyzed with a gamma counter (1470 Wizard, Perkin Elmer).
Laser ablation ICP MS imaging of 195mPt biodistribution in mice tibia
The experimental procedure was carried out as reported previously35. The mice tibias were incubated in neutral buffered formaldehyde for 36 h. Later, tibias were dehydrated in ascending grades of 70% to 100% ethanol and embedded in poly(methyl methacrylate) (pMMA) resin, freshly prepared by mixing 600 mL of methyl methacrylate monomer (Acros Organics BVBA, Geel, Belgium), 60 ml dibutyl phthalate (Merck KGaA, Darmstadt, Germany) and 1.25 g perkadox®16 (Aldrich, Netherlands). The polymerization was followed by cutting serial horizontal sections (perpendicular to the tibia sample) of 5 µm thickness within the trabecular region of interest using an RM2155 microtome with a TC 65 blade (Leica Microsystems GmbH, Wetzlar, Germany). Microscopic images were obtained using an inverted fluorescence/bright field microscope (BZ-9000, Keyence Deutschland GmbH, Neu-Isenburg, Germany). For image recording and processing, the software BZ-II Viewer and BZ-II Analyzer were used, respectively. To calculate the percentage of platinum co-localized with calcium, the background of the image and all pixels without hard bone tissue were excluded from the calculations. A pixel is considered to be background if its calcium intensity is below 15%. The platinum concentrations of the remaining pixels were then added up and divided by the sum of the entire platinum image to yield the percentage of platinum co-localized with calcium35.
Zebrafish care and handling
The zebrafish (Danio rerio) strains were kept under standard conditions (28 °C in E3 buffer) until 48 hours post fertilization (hpf) as described previously56. All animal experiments were conducted at larval stages before the point of independent feeding and were in agreement with the animal protection law (Tierschutzgesetz).
Phenotypic assessment of drug-treated embryos
48 hpf wildtype embryos were separated into 6 groups (n = 20 embryos/group), i.e., control (untreated) and embryos treated with 5 µM, 10 µM, 50 µM, and 100 µM Pt-BP as well as 30 µM cisplatin. Both types of compounds were added externally to the medium and the embryos were incubated at 33 °C for the duration of the experiment. The embryos were assessed each day for phenotypic toxicity until 2 days post treatment (48 hpt) and imaged live in an Olympus MVX10 microscope.
Ototoxicity assay
The lateral line neuromast hair cells in embryos were stained with a vital dye to analyse the loss of hair cells after Pt-BP treatment. Cisplatin was used as a control, as it was known to affect hair cells in humans. The fluorescent dye 2-[4-(dimethylamino)styryl]-N-ethylpyridinium iodide (DASPEI) [Molecular Probes, Eugene, OR] was used to stain hair cells in the neuromast as described previously57. Larvae were incubated in embryo medium containing 0.005% DASPEI for 15 min, anesthetized in Tricaine MS222 (10 lg/ml) for 5 min, rinsed once in fresh embryo medium, and imaged using Vertebrate automated screening technology (VAST, Union Biometrica), combined with high-speed and super-resolution Zeiss cell observer spinning disk confocal system. The quantification of number of neuromast hair cells in the treated embryos was done manually with confocal microscopy (Zeiss LSM780).
Statistical analysis
All results except ratios are depicted as mean ± standard deviation. The statistical analyses were performed using GraphPad Prism (version 6.0) software. Two-way analysis of variance (ANOVA) with a Bonferroni (multiple comparisons) post-hoc test was used to determine the differences among the two groups. For ratios, paired t-test was used to determine the differences among the two groups. For the ototoxicity assay, one-way analysis of variance (ANOVA) was performed followed by the Dunnett’s method for multiple comparisons.
References
Chantrain, C. F., Feron, O., Marbaix, E. & DeClerck, Y. A. Bone Marrow Microenvironment and Tumor Progression. Cancer Microenviron. 1, 23–35, https://doi.org/10.1007/s12307-008-0010-7 (2008).
Ren, G., Esposito, M. & Kang, Y. Bone metastasis and the metastatic niche. J. Mol. Med. 93, 1203–1212, https://doi.org/10.1007/s00109-015-1329-4 (2015).
Lee, R. J., Saylor, P. J. & Smith, M. R. Treatment and prevention of bone complications from prostate cancer. Bone 48, 88–95, https://doi.org/10.1016/j.bone.2010.05.038 (2011).
Zhang, H. et al. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: A systematic review of the real world data. J. Bone Oncol. 11, 38–50, https://doi.org/10.1016/j.jbo.2018.01.004 (2018).
Body, J. J. et al. A real-world study assessing the use of bone-targeted agents and their impact on bone metastases in patients with prostate cancer treated in clinical practice in Europe. J. Bone Oncol. 14, 100212, https://doi.org/10.1016/j.jbo.2018.100212 (2019).
Gnant, M. & Hadji, P. Prevention of bone metastases and management of bone health in early breast cancer. Breast Cancer Res. 12, 216 (2010).
Cathomas, R. et al. Management of bone metastases in patients with castration-resistant prostate cancer. Urol. Int. 92, 377–386, https://doi.org/10.1159/000358258 (2014).
von Moos, R., Costa, L., Ripamonti, C. I., Niepel, D. & Santini, D. Improving quality of life in patients with advanced cancer: Targeting metastatic bone pain. Eur. J. Cancer 71, 80–94, https://doi.org/10.1016/j.ejca.2016.10.021 (2017).
Fernandes, R. et al. Future directions for bone metastasis research - highlights from the 2015 bone and the Oncologist new updates conference (BONUS). J. Bone Oncol. 5, 57–62, https://doi.org/10.1016/j.jbo.2016.02.004 (2016).
Price, T. T. et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 8, 340ra373, https://doi.org/10.1126/scitranslmed.aad4059 (2016).
Jadvar, H. Targeted Radionuclide Therapy: An Evolution Toward Precision Cancer Treatment. AJR Am. J. Roentgenol. 209, 277–288, https://doi.org/10.2214/AJR.17.18264 (2017).
Parker, C., Heidenreich, A., Nilsson, S. & Shore, N. Current approaches to incorporation of radium-223 in clinical practice. Prostate Cancer Prostatic Dis. 21, 37–47, https://doi.org/10.1038/s41391-017-0020-y (2018).
Morris, M. J. et al. Effects of radium-223 dichloride (Ra-223) with docetaxel (D) vs D on prostate-specific antigen (PSA) and bone alkaline phosphatase (bALP) in patients (pts) with castration-resistant prostate cancer (CRPC) and bone metastases (mets): A phase 1/2a clinical trial. J. Clin. Oncol. 33, 5012–5012, https://doi.org/10.1200/jco.2015.33.15_suppl.5012 (2015).
Bouman-Wammes, E. W. et al. Bone-Targeting Radiopharmaceuticals as Monotherapy or Combined With Chemotherapy in Patients With Castration-Resistant Prostate Cancer Metastatic to Bone. Clin Genitourin Cancer, https://doi.org/10.1016/j.clgc.2018.11.014 (2018).
Vokes, E. E., Choy, H., Gandara, D. & Mattson, K. Adjuvant and neoadjuvant treatments for NSCLC. Lung Cancer 38, 29–35, https://doi.org/10.1016/S0169-5002(02)00169-1 (2002).
Nabell, L. & Spencer, S. Docetaxel with concurrent radiotherapy in head and neck cancer. Semin. Oncol. 30, 89–93, https://doi.org/10.1053/j.seminoncol.2003.11.017 (2003).
Wardman, P. Chemical Radiosensitizers for Use in Radiotherapy. Clin. Oncol. 19, 397–417, https://doi.org/10.1016/j.clon.2007.03.010 (2007).
Howell, R. W. et al. Radiotoxicity of platinum-195m-labeled trans-platinum (II) in mammalian cells. Radiat. Res. 140, 55–62 (1994).
Areberg, J., Norrgren, K. & Mattsson, S. Absorbed doses to patients from 191Pt-, 193mPt- and 195mPt-cisplatin. Appl. Radiat. Isotopes 51, 581–586, https://doi.org/10.1016/S0969-8043(99)00082-2 (1999).
Areberg, J., Wennerberg, J., Johnsson, A., Norrgren, K. & Mattsson, S. Antitumor effect of radioactive cisplatin (191Pt) on nude mice. Int. J. Radiat. Oncol. Biol. Phys. 49, 827–832, https://doi.org/10.1016/S0360-3016(00)01419-X (2001).
Lange, R. C., Spencer, R. P. & Harder, H. C. Synthesis and distribution of a radiolabeled antitumor agent: cis-diamminedichloroplatinum. II. J. Nucl. Med. 13, 328–330 (1972).
Norrgren, K. et al. Comparative Renal, Hepatic, and Bone Marrow Toxicity of Cisplatin and Radioactive Cisplatin (191Pt) in Wistar Rats. Cancer Biother. Radiopharm. 21 (2006).
Bodnar, E. N., Dikiy, M. P. & Medvedeva, E. P. Photonuclear production and antitumor effect of radioactive cisplatin (195mPt). J. Radioanal. Nucl. Chem. 305, 133–138, https://doi.org/10.1007/s10967-015-4053-1 (2015).
Aalbersberg, E. A. et al. Preclinical imaging characteristics and quantification of Platinum-195m SPECT. Eur. J. Nucl. Med. Mol. Imaging 44, 1347–1354, https://doi.org/10.1007/s00259-017-3643-2 (2017).
Jadvar, H. & Colletti, P. M. (18)F-NaF/(223)RaCl2 theranostics in metastatic prostate cancer: treatment response assessment and prediction of outcome. Br. J. Radiol. 91, 20170948, https://doi.org/10.1259/bjr.20170948 (2018).
Kruger, K. et al. Platinum-induced kidney damage: Unraveling the DNA damage response (DDR) of renal tubular epithelial and glomerular endothelial cells following platinum injury. Biochim. Biophys. Acta 1853, 685–698, https://doi.org/10.1016/j.bbamcr.2014.12.033 (2015).
Amable, L. Cisplatin resistance and opportunities for precision medicine. Pharmacol. Res. 106, 27–36, https://doi.org/10.1016/j.phrs.2016.01.001 (2016).
Johnstone, T. C., Suntharalingam, K. & Lippard, S. J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 116, 3436–3486, https://doi.org/10.1021/acs.chemrev.5b00597 (2016).
Margiotta, N. et al. Bisphosphonate complexation and calcium doping in silica xerogels as a combined strategy for local and controlled release of active platinum antitumor compounds. Dalton Trans, 3131–3139, https://doi.org/10.1039/b705239a (2007).
Palazzo, B. et al. Biomimetic Hydroxyapatite–Drug Nanocrystals as Potential Bone Substitutes with Antitumor Drug Delivery Properties. Adv. Funct. Mater. 17, 2180–2188, https://doi.org/10.1002/adfm.200600361 (2007).
Iafisco, M. et al. Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. Journal of Materials Chemistry 19, https://doi.org/10.1039/b914379c (2009).
Farrell, K. B., Karpeisky, A., Thamm, D. H. & Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 9, 47–60, https://doi.org/10.1016/j.bonr.2018.06.007 (2018).
Bussard, K. M., Gay, C. V. & Mastro, A. M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 27, 41–55, https://doi.org/10.1007/s10555-007-9109-4 (2008).
Chen, Y.-C., Sosnoski, D. M. & Mastro, A. M. Breast cancer metastasis to the bone: mechanisms of bone loss. Breast Cancer Res. 12, 215, https://doi.org/10.1186/bcr2781 (2010).
Crone, B. et al. Quantitative imaging of platinum-based antitumor complexes in bone tissue samples using LA-ICP-MS. J. Trace Elem. Med. Biol. 54, 98–102, https://doi.org/10.1016/j.jtemb.2019.04.011 (2019).
Stornetta, A., Zimmermann, M., Cimino, G. D., Henderson, P. T. & Sturla, S. J. DNA Adducts from Anticancer Drugs as Candidate Predictive Markers for Precision Medicine. Chem. Res. Toxicol. 30, 388–409, https://doi.org/10.1021/acs.chemrestox.6b00380 (2017).
Aston, W. J. et al. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer 17, 684, https://doi.org/10.1186/s12885-017-3677-7 (2017).
Hudson, B. & Loots, G. G. In Genetics of Bone Biology and Skeletal Disease (eds. Rajesh, V. Thakker, Michael, P. Whyte, John A. Eisman & Takashi, Igarashi) 101–121 (Academic Press, 2013).
Caglar, M., Kupik, O., Karabulut, E. & Hoilund-Carlsen, P. F. Detection of bone metastases in breast cancer patients in the PET/CT era: Do we still need the bone scan? Rev. Esp. Med. Nucl. Imagen Mol. 35, 3–11, https://doi.org/10.1016/j.remn.2015.08.006 (2016).
Raynor, W. Y., Al-Zaghal, A., Zadeh, M. Z., Seraj, S. M. & Alavi, A. Metastatic Seeding Attacks Bone Marrow, Not Bone: Rectifying Ongoing Misconceptions. PET. Clin. 14, 135–144, https://doi.org/10.1016/j.cpet.2018.08.005 (2019).
Hillner, B. E. et al. 18F-fluoride PET used for treatment monitoring of systemic cancer therapy: results from the National Oncologic PET Registry. J. Nucl. Med. 56, 222–228, https://doi.org/10.2967/jnumed.114.150391 (2015).
Rao, L. et al. 18F-Labeled NaF PET-CT in Detection of Bone Metastases in Patients With Preoperative Lung Cancer. Med. 95, e3490, https://doi.org/10.1097/MD.0000000000003490 (2016).
Margiotta, N. et al. Platinum-bisphosphonate complexes have proven to be inactive chemotherapeutics targeted for malignant mesothelioma because of inappropriate hydrolysis. J. Inorg. Biochem. 105, 548–557, https://doi.org/10.1016/j.jinorgbio.2010.12.011 (2011).
Liu, L. et al. A new approach to reduce toxicities and to improve bioavailabilities of platinum-containing anti-cancer nanodrugs. Sci. Rep. 5, 10881, https://doi.org/10.1038/srep10881 (2015).
Chen, H., Zhou, X., Fujita, H., Onozuka, M. & Kubo, K. Y. Age-related changes in trabecular and cortical bone microstructure. Int. J. Endocrinol. 2013, 213234, https://doi.org/10.1155/2013/213234 (2013).
Berish, R. B., Ali, A. N., Telmer, P. G., Ronald, J. A. & Leong, H. S. Translational models of prostate cancer bone metastasis. Nat. Rev. Urol. 15, 403–421, https://doi.org/10.1038/s41585-018-0020-2 (2018).
Cui, J. J. et al. Gene-gene and gene-environment interactions influence platinum-based chemotherapy response and toxicity in non-small cell lung cancer patients. Sci. Rep. 7, 5082, https://doi.org/10.1038/s41598-017-05246-8 (2017).
Buck, L. M., Winter, M. J., Redfern, W. S. & Whitfield, T. T. Ototoxin-induced cellular damage in neuromasts disrupts lateral line function in larval zebrafish. Hear. Res. 284, 67–81, https://doi.org/10.1016/j.heares.2011.12.001 (2012).
Hung, G. Y. et al. Cisplatin exposure impairs ionocytes and hair cells in the skin of zebrafish embryos. Aquat. Toxicol. 209, 168–177, https://doi.org/10.1016/j.aquatox.2019.02.006 (2019).
Margiotta, N. et al. Synthesis, characterization, and cytotoxicity of dinuclear platinum-bisphosphonate complexes to be used as prodrugs in the local treatment of bone tumours. Dalton Trans, 10904–10913, https://doi.org/10.1039/b919721d (2009).
Farbod, K. et al. Controlled Release of Chemotherapeutic Platinum-Bisphosphonate Complexes from Injectable Calcium Phosphate Cements. Tissue Eng. Part. A 22, 788–800, https://doi.org/10.1089/ten.TEA.2016.0001 (2016).
Farbod, K. The use of bisphosphonates for bone-specific drug delivery, Radboud University Medical Center, (2016).
Curci, A. et al. Novel Kiteplatin Pyrophosphate Derivatives with Improved Efficacy. Inorg. Chem. 56, 7482–7493, https://doi.org/10.1021/acs.inorgchem.7b00931 (2017).
van der Have, F. et al. U-SPECT-II: An Ultra-High-Resolution Device for Molecular Small-Animal Imaging. J. Nucl. Med. 50, 599–605, https://doi.org/10.2967/jnumed.108.056606 (2009).
Heskamp, S. et al. Noninvasive Imaging of Tumor PD-L1 Expression Using Radiolabeled Anti-PD-L1 Antibodies. Cancer Res. 75, 2928–2936, https://doi.org/10.1158/0008-5472.CAN-14-3477 (2015).
Brand, M., Granato, M., Nüsslein-Volhard, C., Nusslein-Volhard, C. & Dahm, R. Zebrafish: A practical approach. Keep. Rais. zebrafish 7, 39 (2002).
Harris, J. A. et al. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 4, 219–234, https://doi.org/10.1007/s10162-002-3022-x (2003).
Acknowledgements
The authors would like to thank Bianca Lemmers-van de Weem for the practical assistance with animal studies, Paul van der Ven for the practical assistance with ICP-MS measurements, Peter Nobels for the practical assistance with High resolution ICP-MS measurements, and Natasja Wilhelmina Maria van Dijk for the practical assistance in tissue processing. In addition, the authors thank Dr. Stefan Hans for support with the zebrafish ototoxicity assay and Dr. Guruchandar Arulmozhivarman for assistance with VAST imaging at the in vivo testing facility of the CRTD. The experiments reported here also featured in the doctoral thesis of K.F. The authors are grateful for the funding support from the NIRM, Netherlands Institute for Regenerative Medicine, and the Radboud Institute for Molecular Life Science (RIMLS, grant #014-057), Radboud University Nijmegen Medical Center.
Author information
Authors and Affiliations
Contributions
R.A.N., M.I., N.M., U.K., J.J.J.P.B. and S.C.G.L. designed the research. K.C.S., A.C., N.M. and O.C.B. provided the materials. K.F., A.C. and N.M. established the new synthesis protocol of Pt-BP. A.C. and N.M. performed NMR experiment. R.A.N. and K.F. performed the bone-seeking in vivo experiment. R.A.N. performed the synthesis and the biodistribution study of 195mPt-BP. R.A.N. and S.H. analyzed the µSPECT data for quantification of 195mPt distribution. R.A.N., L.S. and B.C. performed the LA-ICP-MS imaging experiments. N.A., M.B. and M.B. performed the zebrafish experiment and analyzed data. R.A.N., K.F., J.J.J.P.B., U.K. and S.C.G.L. analyzed data. R.A.N., J.J.J.P.B. and S.C.G.L. wrote the manuscript. All authors read and corrected the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nadar, R.A., Farbod, K., der Schilden, K.Cv. et al. Targeting of radioactive platinum-bisphosphonate anticancer drugs to bone of high metabolic activity. Sci Rep 10, 5889 (2020). https://doi.org/10.1038/s41598-020-62039-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-62039-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.