Abstract
Emotional contagion, the ability to feel what other individuals feel without necessarily understanding the feeling or knowing its source, is thought to be an important element of social life. In humans, emotional contagion has been shown to be stronger in women than men. Emotional contagion has been shown to exist also in rodents, and a growing number of studies explore the neural basis of emotional contagion in male rats and mice. Here we explore whether there are sex differences in emotional contagion in rats. We use an established paradigm in which a demonstrator rat receives footshocks while freezing is measured in both the demonstrator and an observer rat. The two rats can hear, smell and see each other. By comparing pairs of male rats with pairs of female rats, we found (i) that female demonstrators froze less when submitted to footshocks, but that (ii) the emotional contagion response, i.e. the degree of influence across the rats, did not depend on the sex of the rats. This was true whether emotional contagion was quantified based on the slope of a regression linking demonstrator and observer average freezing, or on Granger causality estimates of moment-to-moment freezing. The lack of sex differences in emotional contagion is compatible with an interpretation of emotional contagion as serving selfish danger detection.
Similar content being viewed by others
Introduction
Affective empathy, i.e. feeling what another feels while knowing that the other person’s affective state is the source of our own affective state1,2, has often been reported to show gender differences in humans3,4,5, with women more affected by the emotions of others. Many believe that empathy evolved in the context of parental care, where feeling the distress of offspring motivates nurturing behavior and thereby increases Darwinian fitness2,6,7. If empathy serves maternal care, one may predict empathy to be stronger in females in species that parental investment is mainly provided by females.
Emotional contagion is thought of as an evolutionary predecessor to empathy6,7,8,9. Different from empathy, emotional contagion is a transfer of emotion from one individual to another without requiring cognitive understanding, perspective-taking or the intention to help. The term emotional contagion can be traced back to the German ‘Stimmungsuebertragung’ introduced by Konrad Lorenz to refer to cases in which witnessing a conspecific in a particular emotion, expressed via movements and sounds, triggers a similar emotion in the witness (“der Anblick des Artgenossen in bestimmten Stimmungen, die sich durch Ausdrucksbewegungen und -laute äußern können, im Vogel selbst eine ähnliche Stimmung hervorruft”10). Similar to empathy, in humans, there is some evidence that emotional contagion is more pronounced in women4,11,12,13, and female babies are more likely to cry and cry for longer when hearing another baby cry14,15.
Mounting experimental evidence suggests that rats and mice show signs of emotional contagion16,17,18,19,20,21,22,23. Would they also show sex differences in emotional contagion with females showing more contagion than males? Emotional contagion can be quantified systematically in rodents using designs in which one demonstrator animal receives a footshock, and the freezing of another observer that witnesses the event is found to be increased, suggesting that the distress of the shocked demonstrator was transferred to the observer16,17,21,24,25,26,27,28. We have recently introduced ways to quantify emotional transfer in this paradigm by leveraging Bayesian statistics and Granger causality28. Here we use these quantification methods to investigate whether emotional contagion is stronger in female Long Evans rats compared to males. With increasing interests in the biological and neural basis of emotional contagion21,22,27,29,30,31, exploring sex differences is important. This is particularly true in a paradigm involving the nociceptive system and freezing as behavioral read-out because profound sex differences exist in the biology of the nociceptive system32, and female rats have been shown to be generally more active than males and respond to shocks with less freezing33,34.
To date, the majority of studies investigating sex differences on emotional contagion have been conducted in mice23,25,26,35,36,37,38,39. In fear observation paradigms, in which the response of an observer mouse is measured while it witnesses another experiencing a negative stimulus (generally a footshock), evidence from mice is contradictory: while Keum et al. (2016), Sanders et al. (2013) and Chen et al. (2009) find no effect of sex, Pisansky et al. (2017) finds that 1) females mice have a greater amount of emotional contagion as measured by higher amounts of freezing, 2) in contrast to males, familiarity does not play a role in female mice, and 3) this response is modulated by oxytocin. These opposing effects between studies could be due to differences in the shocking protocols (e.g., a much longer testing session in Pisansky compared to the other three studies) and the freezing quantification method. In paradigms measuring emotional contagion through pain hypersensitivity, female mice display a larger amount of socially transferred pain hypersensitivity compared to males38. Noteworthy, in these types of paradigms, the same pattern of the familiarity effect observed in Pisansky has been reported23,36: while female mice have an equivalent response when with a familiar and an unfamiliar animal, males show a reduced effect when tested with an unfamiliar conspecific. However, this effect is reversed in paradigms measuring approach to conspecifics in distress35: female mice approach distressed cagemates more than unfamiliar animals, and males do not differentiate between familiar vs unfamiliar demonstrators. This approach effect seems to be conserved across rodents as the same phenomenon is observed in rats40.
Overall, in rats, there is a much smaller number of studies that have investigated the effect of sex on emotional contagion or in related paradigms such as social buffering (e.g. presence of an affiliative conspecific mitigates stress responses in a subject), social avoidance learning (an indirect proxy measure for emotional contagion41) or prosociality (e.g. helping). No studies have measured the effect of sex in fear observation paradigms; inconclusive results have been reported in a study investigating social buffering (sex effects that go in opposite direction at the behavior and endocrine level42) and one study finds subtle sex differences on two-way avoidance learning41. Lastly, only one study in rodents43 has reported sex effects in a paradigm measuring prosociality in rats, where they find that females are more likely to help a conspecific in distress than males, supporting the notion that females are more empathic than males. The sparsity of studies in rats and the non-converging results in mice motivated us to apply our new analytical approach to investigate sex differences in emotional contagion in rats.
To compare emotional contagion in males and female rats, we thus harnessed a paradigm developed in our lab in which a shock-experienced observer rat interacts through a perforated transparent divider with a demonstrator rat receiving footshocks. Observers and demonstrators never exchanged roles and they were familiarized with each other before testing. We then quantified the freezing behavior of both animals (10 male observer-demonstrator dyads and 9 female dyads in this study) during an initial 12 min baseline period and a 12 min test period in which the demonstrator received 5 footshocks (1.5 mA, 1 s each, ISI: 120 or 180 s, Fig. 1). We have previously shown that there is mutual influence across demonstrators and observers, with the distress of the shocked demonstrators triggering freezing in observers and individual differences in observer reaction influencing back how much demonstrators freeze28. Interestingly, this mutual influence was not affected by the degree of familiarity across individuals28. Based on this finding, we have suggested that this emotional transfer might have less to do with empathic distress on behalf of the demonstrator, and more to do with using the emotional state of others as a source of information about dangers to the self28. Although we use the term emotional contagion for this phenomenon, because a witness ‘catches’ the emotion of another animal, the term emotional communication or emotional information transfer may arguably be more appropriate, as used in the context of cross-species eavesdropping28,44,45,46. In addition to (i) comparing the average freezing level of observers across the two sexes, here we therefore additionally (ii) compare emotional contagion in terms of the relationship between the freezing of observers and demonstrators in terms of average freezing during the shock period using Bayesian regression analyses and (iii) in terms of second-to-second freezing influences using Granger causality28.
Timeline of the emotional contagion test. Following the first day of habituation, in the shock pre-exposure session, the observer animals were exposed to footshocks alone in a context that is different from the test apparatus (day 2). The pre-exposure session was followed by two more days of habituation (day 3 & 4) to reduce contextual fear in the contagion test session. On day 5, demonstrator-observer dyads were placed in the setup for a total of 24 min. After a 12 min baseline period which is identical to the habituation session, the demonstrators received 5 footshocks (each 1.5 mA, 1 s long) during the 12 min shock period. The inter-shock intervals were either 2 or 3 min. Footshocks of 1.5 mA triggered squeaks in the audible range that are considered a highly specific indicator of pain58, 22 kHz vocalizations, jumping and pain grimaces59 in the demonstrators of both sexes, see27 for details.
Given that in the past we had run an experiment with only females16, and later several experiments with only males17,27,28, and observed higher levels of freezing in both male observers and male demonstrators than in our original female sample, we expected that females would show reduced levels of freezing compared to males in this experiment. However, given that female demonstrators froze less than male demonstrators, it remains unclear whether the reduced freezing of female observers compared to male observers represent a reduced transfer of emotional information, or simply a sex-independent transfer of sex-dependent demonstrator freezing. Stronger emotional transfer in males would be in contrast to the human literature in which females show higher empathy than males, and the notion that emotional contagion serve child care6,7. Sex-independent transfer, on the other hand, would dovetail with the lack of an effect of familiarity in this paradigm, in that it would align with the notion that emotional contagion serves as a selfish source of information about one’s own danger28.
Results
Average freezing is higher in male rats
Figure 2A shows the group freezing data of observers and demonstrators of both sexes. A test of normality of the freezing variables separately for the two sexes revealed that freezing during the shock epoch is normally distributed for both sexes and roles (Shapiro-Wilk47, all W > 0.9, p > 0.3). Unfortunately, freezing during baseline deviates from normality for both sexes and roles (Shapiro-Wilk, all W < 0.78, p < 0.05). Accordingly, parametric tests including the baseline should be interpreted with caution, and are supplemented by non-parametric tests where possible.
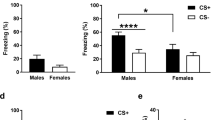
Emotional Contagion as a function of sex. (A) Freezing percent during the baseline (open violins) and the shock period (filled violins) for male (blue) and female (purple) rats, with observer data on the left and demonstrator data on the right. The black bar represents the mean, the box ± SEM. (B) Observer freezing as a function of demonstrator freezing during the shock period, including linear regression lines and their 95% confidence intervals. (C) Demonstrator freezing as a function of observer freezing during the shock period including linear regression lines and their 95% confidence intervals. (D) Granger causality F values in the dem- > obs (left) and obs- > dem (right) direction during the shock period. For all panels: *:p < 0.05, **:p < 0.01, ***:p < 0.001 in two-tailed t-test; ## = p < 0.005 in Wilcoxon test. Other conventions are the same as in (A). We use violin plots here, because some of the data is not normally distributed, and mean and s.e.m. therefore do not provide a full picture of the distribution of the data.
For observers a 2 Sex (male vs female) x 2 Epoch (baseline vs shock) ANOVA revealed a main effect of Epoch (F(1,17) = 56, p < 0.001, BFincl = 874958), a trend for Sex (F(1,17) = 3.86, p = 0.066, BFincl = 1.3) but no interaction (F(1,17) = 1.002, p = 0.33, BFincl = 1.3). For those less familiar with Bayesian statistics, BFincl refers to the Bayes factor for including the effect and is calculated as the likelihood of models including a certain effect divided by that of models excluding that effect. BFincl values above 3 are considered moderate evidence that including the effect improves the model, whilst BFincl below 1/3 are considered moderate evidence that including the effect worsens the model. Intermediate BF values are considered inconclusive48. So here, the Bayesian ANOVA provides very strong evidence for the effect of Epoch, but with regard to the effect of Sex and interaction, the data is inconclusive, in that the variance is such that the data is about equally likely with models with or without these effects. Paired tests comparing observers’ freezing during the baseline and the shock period confirm that witnessing the demonstrator receive shocks increases freezing compared to baseline in both sexes examined individually (Wilcoxon W(9) = 0, p = 0.002 for males; W(8) = 0, p = 0.004 for females; Fig. 2A). We then compared the freezing of the male versus the female observers during the shock period. Considering the findings from previous research, we had hypothesized lower freezing in females compared to male observers. A one-tailed t-test on the observer freezing confirmed this prediction (t(17) = 1.8, p < 0.044), with a large effect size with males freezing 1.5 times as much as females (Cohen d = 0.8, Fig. 2A).
For demonstrators a 2 Sex (male vs female) x 2 Epoch (baseline vs shock) on freezing also revealed a main effect of Epoch (F(1,17) = 115, p < 0.001, BFincl = 1E10), but also revealed a significant main effect for Sex (F(1,17) = 19, p < 0.001, BFincl = 208) and an interaction (F(1,17) = 11, p = 0.004, BFincl = 103), due to a larger increase in freezing following the shocks in males. A one-tailed t-test again confirms that during the shock epoch, male demonstrators froze more than female demonstrators (t(17) = 3.97, p < 0.001) with an even larger effect size than for the observers: males froze twice as much as females (Cohen d = 1.8, Fig. 2A).
For both observers and demonstrators, males thus froze more than females. That the effect size was more pronounced for demonstrators (d = 1.8) than observers (d = 0.8) raises the question of whether the smaller sex difference in observers is a downstream result of the larger sex difference in demonstrators, a question we will address in the next section.
Demonstrator freezing level, independently of sex, is the best predictor of observer freezing
To understand whether sex differences in observers’ freezing during the shock epoch (obsf) were due to differences in the demonstrators’ freezing (demf) during the shock period alone we performed an ANCOVA, which tests whether after regressing out the individual differences in demonstrator freezing (demf), there is a residual main effect of observer sex, or an interaction of demf and observer sex. This was done using a traditional (frequentist) ANCOVA and a Bayesian ANCOVA with 2 sex (male vs female) x demf, see Table 1A.
The frequentist analysis revealed a significant influence of demf confirming that the observer freezing reflects the demonstrator freezing but neither a significant main effect of sex or interaction of sex * demf were detected. This shows, that once the difference in demonstrator freezing have been accounted for, sex fails to explain additional variance. This suggests, that difference in observer freezing can be most parsimoniously explained by knowing the level of freezing of the demonstrator, independently of sex. In Fig. 2B, this is apparent in the linear regression lines that have confidence intervals of the slope that overlap. In addition, Fig. 2B shows how when considering females paired with the higher-freezing female demonstrators (on the right of the figure), the confidence interval (pink) overlaps considerably with the male data. This suggests that the differences in demonstrator freezing simply acted as distinct input onto a transmission function (i.e. slope and offset) that is the same independently of the sex of the observer. Given that female demonstrators reacted to the shocks with less freezing, this simply transforms into the group difference in observer freezing we observe.
A non-significant main effect of sex or interaction with sex could reflect evidence that there is no effect of sex (evidence of absence), or that our study was underpowered and cannot speak for or against the absence of a sex difference. To shed light on this issue, we performed a Bayesian ANCOVA that explains observer freezing using competing models with or without sex as a factor (Table 1B). A model only considering demf is the best model by a margin (P(M|data), Table 1B), and an analysis of effects provides strong evidence for an effect of demf (BFincl = 19), confirming that the demonstrator freezing strongly determines observer freezing. The analysis also shows that the evidence leans towards the absence of an effect of sex, be it as a main effect (BFincl = 0.471) or sex*demf interaction (BFincl = 0.463), showing that the data is over twice as likely in models without these factors than in models with them (BFincl = 1/2 = 0.5 indicates that models without the factor are twice as likely as models with, and values below 0.5 show that models without the effect are more then twice as likely). Indeed, comparing the probability of different models given the data (Table 1B, column P(M|data)) shows a full model in which sex and its interaction are included (sex + demf + sex * demf) is over 5 times less likely than one only including demf. Altogether this shows that our data is best explained by a model that considers the level of freezing of the demonstrator (which is different for male and female demonstrators, as shown above) but ignores the sex of the animals involved.
To further characterize the relation between observers’ and demonstrators’ freezing in the two sexes, we performed separate Bayesian regressions for males and females. This gave highly overlapping posterior estimates for the regression weight for obsf = β*demf + intercept, with β for females having mean = 0.44 (95% credibility interval CI = [0.0,1.09]) and male having mean = 0.36(95%CI = [−0.5,1.9]). Figure 2B illustrates this as the similarity in slope.
In our past work28, we have shown that differences in the freezing level of the observer can influence back the freezing level of the demonstrator, a phenomenon akin to social buffering, in that observers that showed unusually low levels of freezing due to inactivation of the ACC reduced freezing levels in the demonstrators. To explore whether there might be a sex difference in this influence of obsf on demf, we performed a frequentist ANCOVA and a Bayesian model comparison between different models explaining freezing of the demonstrators as a function of sex, freezing of observers (obsf) and their interaction (sex*obsf, Table 2). The frequentist ANCOVA showed significant main effects (Table 2A). The Bayesian model comparison found that including sex and observers’ freezing in an additive model (obsf + sex) best describes the data (Table 2B). There was strong evidence for a contribution of sex in predicting demonstrator freezing (BFincl = 14.712), with the females freezing less to the shock than the males. There was also strong evidence for a contribution of observer freezing (BFincl = 18.390). However, there was only a trend and anecdotal evidence for including an interaction effect (BFincl = 2.804) indicating that if there was a sex difference in the feedback from the observer to the demonstrator, we would need a larger group to find robust evidence for such an effect. This is evident in the similarity in slope across the sexes in Fig. 2C.
No sex difference in granger-causality across the animals
To further explore whether males and females differ in the temporal coupling of the freezing between demonstrators and observers, we performed Granger causality analyses (Fig. 2D). Unlike the other analyses that explore the average freezing over the 12 min of the shock period, the Granger causality analyses explore the relation between the second-to-second freezing of demonstrators and observers. Specifically, it examines if past demonstrator freezing can explain present observer freezing (to quantify influences in the dem → obs direction), and if past observer freezing can explain present demonstrator freezing (to quantify influences in the obs → dem direction). Higher G-causality values (i.e. Granger F values) indicate higher temporal coupling of the behavior of the two animals in a pair, and thereby stronger social sensitivity to the behavior of the other. A Granger analysis considering all animals (irrespective of sex) revealed significant information flow in both directions (dem → obs Granger F = 0.039, p < 0.0001; obs → dem Granger F = 0.034, p < 0.0001). Because the G-causality values were not normally distributed (Shapiro W < 0.76, p < 0.05), we used non-parametric tests to compare the sexes. We found no significant sex difference in Granger causality in either direction (Mann-Whitney U, dem → obs, U(17) = 38, p = 0.6; obs → dem, U(17) = 41, p = 0.78; Fig. 2D). Bayesian Mann-Whitney U tests revealed that in both directions, the evidence leans towards the null hypothesis (i.e. no sex difference), but with limited evidence strength: in dem → obs direction, the Bayes factor in favor of the null hypothesis BF01 = 2.4, in the obs → dem direction BF01 = 2.1.
A trade-off between rearing and freezing
The finding that the female demonstrators froze less than their male counterparts raises the question of whether they reacted to the shocks using an alternative strategy. It has often been described that individual and sex differences exist in the propensity to react to danger with escape vs freezing33. We thus explored whether females reared (including attempts to climb out of the box) more than their male counterparts (Fig. 3A). All rearing data were normally distributed (Shapiro W > 0.86, p > 0.1) except for male observer rearing during the shock epoch.
Rearing/climbing as a function of sex. (A) distribution of rearing and climbing. (B) The trade-off of rearing and climbing during the shock epoch for demonstrators. All conventions as in Fig. 2. ## = p < 0.01 in the Wilcoxon test.
All groups showed reduced rearing/climbing following the shocks (Fig. 3A). To explore if that reduction is sex-dependent, we performed mixed frequentist ANOVAs separately for observer rearing and demonstrator rearing, including 2 Sexes (male vs female) x 2 Epochs (baseline vs shock). We found main effects of Epoch in both cases (Obs: F(1,17) = 67, p < 0.001, BFincl = 8.7E7; Dem: F(1,17) = 25, p < 0.001, BFincl = 2478), but no main effect of Sex (Obs: F(1,17) = 2.4, p = 0.141, BFincl = 0.66; Dem: F(1,17) = 0.121, p = 0.732, BFincl = 0.56) or interaction of Sex x Epoch (Obs: F(1,17) = 0.004, p = 0.95, BFincl = 0.66; Dem: F(1,17) = 2.3, p = 0.146, BFincl = 1.25).
We also observed a consistent negative correlation between rearing and freezing in our animals (Fig. 3B). To explore that relationship further, we performed ANCOVAs that explore rearing during the shock period as a function of sex, freezing and sex * freezing, separately for observers and demonstrators. In both cases, the effect of freezing was negative (Obs: F(1,15) = 11.8, p = 0.004, BFincl = 21.5; Dem: F(1,15) = 4.15, p = 0.06, BFincl = 6.07) while the effect of sex (Obs: F(1,15) = 0.12, p = 0.73, BFincl = 0.39, Dem: F(1,15) = 0.30, p = 0.59, BFincl = 0.5) or sex*freezing interaction (Obs: F(1,15) = 0.13, p = 0.72, BFincl = 0.4, Dem: F(1,15) = 0.08, p = 0.78, BFincl = 0.55) were negligible, suggesting a sex-independent trade-off: the more an individual freezes, the less it rears, and vice-versa, confirming the notion that animals that froze less reared more. While most male demonstrators consistently showed high levels of freezing (>50%) and low rearing/climbing (<20%) during the shock period, interindividual differences were salient amongst female demonstrators (Fig. 3B): about half showed a pattern similar to the males, with freezing above 50% and low rearing/climbing, whilst the other half showed a pattern only seen in females, with low levels of freezing (<50%) but higher levels of rearing/climbing (>20%). Hence, while the trade-off is similar across the sexes (similar regression lines), females seem to use a broader range along this trade-off.
No sex differences in freezing to shocks during pre-exposure freezing/rearing
Considering the large sex differences in demonstrator freezing in response to shocks, we also analyzed freezing levels during pre-exposure, where the observers experienced shocks (Fig. 4A). Based on the results from the demonstrator rats, we expected male observers to show about twice as much freezing as female observers. Because freezing in the males during pre-exposure was not normally distributed (Shapiro, W = 0.7, p < 0.001), we used non-parametric tests. Tests revealed significant increases in freezing from baseline to shock in both sexes (Wilcoxon, females: W = 0, p < 0.004, males: W = 0, p < 0.002). While during baseline, males showed significantly higher freezing levels in response to a novel environment (Mann-Whitney U = 13, p = 0.008), no sex-driven differences in freezing were detected during the shock epoch (Mann-Whitney U = 39, p = 0.66). Females during the shock period of this pre-exposure froze much more (mean = 92%; SEM = 3%), than their female demonstrators later did in the contagion test (mean = 37%; SEM = 29%, Mann-Whitney U = 0, p < 0.001).
Freezing level for animals receiving shocks. (A) freezing in observer animals receiving shocks during pre-exposure. (B) freezing of demonstrators receiving shocks during the contagion test of the limited bedding and nesting (LBN) pilot group. The experimental schema above the panels illustrates the shock parameters and the fact that animals were alone in a new context in (A) but together with another animal in a familiar context in (B). ##: Wilcoxon test, p < 0.01, #: Wilcoxon test, p < 0.05. $$: Mann-Whitney U, p < 0.01. **: t-test p < 0.01.
To explore whether observers that froze more during pre-exposure also froze more while observing their demonstrator receive shocks, despite the non-normality of the male pre-exposure freezing, we tentatively performed an ANCOVA on observer freezing during the shock epoch in the emotional contagion test that included sex as a fixed factor and demonstrator freezing and observer freezing during pre-exposure as covariates. The analysis confirmed that demonstrator freezing explains observer freezing during the shock epoch (F(1,15) = 8.3, p = 0.011, BFincl = 22), but neither sex (F(1,15) = 0.08, p = 0.78, BFincl = 0.5) nor pre-exposure freezing do (F(1,15) = 1.23, p = 0.28, BFincl = 0.6).
Finally, the discrepancy between the strong sex effect found in demonstrators during the test situation (Fig. 2A) and its apparent absence during pre-exposure (Fig. 4A) raises the question of whether the sex difference during the contagion test was simply a false positive. To explore this possibility, we drew on data from another group of animals in our experiment. Observer animals in that second group had undergone a limited bedding and nesting (LBN) manipulation as pups and are thus not reported here because they might not be representative of normal behavior. However, demonstrators in that LBN experiment were not submitted to limited bedding and were purchased in adulthood together with those of the main experiment and instead randomly assigned to the control group (that is reported in the method section in this paper). This LBN group can thus be used to confirm the presence or absence of a sex difference in response to shocks in the contagion test. The LBN demonstrators showed the same effect as the demonstrators in our main group (Fig. 4B). Freezing data was normally distributed during the shock period (Shapiro W > 0.9, p > 0.4), but not the baseline period (Shapiro W < 0.71, p < 0.01). During the shock epoch, male LBN demonstrators froze about twice as much as the females during the shock epoch (males: n = 10, mean = 68%, SEM = 4%; females n = 6, mean = 36%, SEM = 9%, t(14) = 3.6, p = 0.003, Cohen’s d = 1.9, BF10 = 13), just as in the main experiment. While this LBN group might still be affected by slightly atypical observer reactions, it confirms that the sex difference found in our main group is robust. To explore whether the difference in freezing between pre-exposure and test could be driven by differences in the shocking protocol (pre-exposure: longer inter-shock intervals and fewer shocks), we also looked at the first 110 s after the first 3 shocks in both sessions. Results showed qualitatively similar results, with significant sex differences in the contagion but not the pre-exposure freezing, indicating that the differences were not due to the different inter-shock intervals.
Discussion
Based on the literature showing less freezing in females in non-social experiments33,34, and our own experiments showing lower levels of freezing in female observers and demonstrators in our emotional contagion tests compared to males (see16 for experiments testing only females rats and17,27,28 for experiments testing only males), we wanted to directly test the effect of sex on our emotional contagion test in one experiment, and expected to find reduced freezing in females compared to males. This expectation was confirmed by our data. However, the specific question of interest was whether there were sex differences in emotional contagion, the degree of affective alignment between two rats. Following up on our recent introduction of dyadic methods, that quantify the relationship between freezing behavior in observers and demonstrators28, here we leveraged these methods to quantify emotional contagion as the strength of the link between the freezing behavior across the two members of each pair. We exploit two methods to do so.
First, we used a regression analysis, which explores the relationship between average freezing of demonstrators and observers during the shock epoch, and found that a model that assumes the same slope for males and females is in fact the best description of our data. That is to say, that male and female observers react with the same amount of freezing to a given degree of freezing of the demonstrator. The sex differences we see in the overall freezing level of observers are thus not due to a difference in emotional contagion (i.e. a difference in sensitivity to demonstrator freezing) per se, but a result of a difference in the amount of freezing displayed by the demonstrator. This is particularly visible in Fig. 2B: female observers paired with those female demonstrators that displayed male-typical levels of freezing ( > 50%) showed a male-typical level of freezing ( > 50%).
Second, we used Granger causality to explore the moment-to-moment relationship between the freezing of the members of each pair. We found reliable, bidirectional evidence of influence across the animals in both male and female dyads, and there was no reliable difference in the strength of this Granger causality. This shows again, that the social transfer of distress, as measured by the relationship in freezing behavior across the members of the dyads was independent of sex.
Overall, because our study had a relatively small sample size (10 male and 9 female dyads), we also consistently used Bayesian statistics to explore whether a lack of significant sex difference in our frequentist approaches was simply due to a lack of power (and thus represents absence of evidence) or provided evidence for the lack of a sex difference. This approach shows that in most cases, Bayes Factors for models considering sex versus the ones not considering sex showed that assuming no effect of sex was about twice as good at predicting the data than models assuming an effect of sex. This means that overall we certainly do not have evidence for an effect of sex, but that we also do not have strong evidence for the absence of an effect of sex. We thus summarize our data as showing that emotional contagion is roughly similar across the sexes in the rats. A larger group size would be necessary to rigorously exclude the presence of even small effects.
In our paradigm, we also quantified how much the demonstrator’s freezing is influenced back by the observer’s freezing. Consistent with our previous work, which has shown that taking the observer’s freezing into account helps predict how much a demonstrator freezes in response to shock16,28, we find in both our regression and Granger causality approach, evidence that the observer’s freezing influences the demonstrator’s freezing. This phenomenon, that bears resemblance with what has often been called social buffering in that it involves a less stressed animal influencing the distress of an animal that receives a shock28,49, was also not sex-dependent in our sample.
In contrast to the lack of a significant sex effect on emotional contagion, we do find a robust sex difference in demonstrator’s freezing, with females freezing half as much as males in response to shocks, a finding confirmed in a second group (Fig. 4B). This difference then feeds onto the observers, which also show, an albeit smaller, sex difference, which can, however, be entirely explained by the sex difference in demonstrator freezing. Intriguingly, we do not find this sex difference during the pre-exposure session, where males and females also received electroshocks. Several differences exist between what observer animals experienced during pre-exposure and what demonstrators experienced during the emotional contagion test. First, the parameters of the footshocks differ (Fig. 4). Observers during pre-exposure experienced 4 footshocks, 0.8 mA, 1 s long, 200–260 s random inter-shock interval, while demonstrators received 5 footshocks, 1.5 mA, 1 s long, 120 or 180 s inter-shock interval. However, we also found no sex difference in the pre-exposure but a sex difference during the emotional contagion test when only testing the first 110 s of the first 3 shocks, suggesting that the difference in the number of footshocks and the inter-shock interval are perhaps unlikely to explain the difference in sex-effects. Shock intensity, however, could play an important role, with both sexes showing similar responses to moderate shocks during pre-exposure but different responses to more intense shocks in the test situation. Second, in pre-exposure animals encounter the pre-exposure context for the first time on the day they receive shocks, while demonstrators in the test context have been habituated for three days to that context. It might be that the sex differences found in the test context reflect the fact that females are more influenced by the three days of safety experienced in this context than males do. Finally, animals during pre-exposure are alone, while demonstrators in the test situation are paired with their cage-mate. Sex differences in social buffering could thus, in principle, create a sex difference in the test situation that is absent during pre-exposure. Whether the strong sex difference in shock-triggered freezing we observe in our test situation is specific to the shock intensity, level of habituation and/or social buffering remains for future experiments to test.
Our study also has a number of important limitations. First, we did not test pairs of mixed sexes, with a female demonstrator paired with a male demonstrator and vice versa. Future experiments should explore if emotional contagion might be stronger within a sex than between sexes. Second, we have only explored a small number of animals in our study, and small sex differences may thus have evaded our analysis. Third, we have only explored emotional contagion in rats that have been pre-exposed to shocks. We have previously shown that pre-exposure of observers to shocks does increases the amount of freezing in observers of both sexes quite dramatically16,28. Whether there might be sex differences in the social transmission of shock-naïve observers might be worth exploring in the future. Fourth, we have only explored the sex difference in Long-Evans rats. In the future, exploring this effect in other rat strains and in mice would be exciting. Fifth, we have not controlled the modality through which information is transferred between rats. Emotional contagion depends on multiple sensory modalities. The sound of the demonstrator’s reactions to the shocks, in particular pain squeaks, play a role in triggering freezing in listeners16,27. Olfactory signals play a role in social buffering50, but may be too slow to trigger the very swift emotional communication in our paradigm27. Vision can also play a significant role in similar paradigms21. Whether sex-effects might be more evident if communication were limited to a specific modality remains to be explored. Finally, we did not stratify our female animals based on the phase of their estrous cycle. Collecting vaginal samples and assessing whether estrous status can explain variance in female emotional contagion would be an exciting avenue for future research.
In summary, we show that although we find significant sex differences in how demonstrators react to a shock, the emotional contagion process, that transmits this reaction across individuals does appear to be sex-independent in Long Evan rats. This echoes two observations we have recently made regarding familiarity in the same paradigm. First, we observed that emotional contagion is independent of how long male observers and demonstrators have been housed together28. Second, we have compared emotional contagion across male Long-Evans and Sprague-Dawley rats. We found that akin to female Long-Evans, Sprague-Dawley male demonstrator rats freeze significantly less to the electroshock. However, the degree of social transmission, as estimated using the slope of a regression or Granger causality, did not depend on the strain. Taken together, this shows that emotional contagion in our paradigm is similar across different sexes, different strains and different levels of familiarity. This finding is compatible with the notion that emotional contagion primarily serves a purpose similar to Eaves-dropping across animals44,45,46, namely the social detection of danger28. If a rat witnesses another rat express distress, this is a valuable, selfish danger signal that the recipient can use as an indicator of danger that should trigger freezing. In this selfish, danger-detection view, one would not necessarily expect that females should show more emotional contagion than males, especially in species in which females are not more vulnerable.
Overall, the lack of a behavioral sex-effect on emotional contagion we find here thus does not lead us to question whether the neuroscience work done to uncover the neural basis of emotional contagion in male rodents21,27,29,30,31 also applies to females. However, similar behavioral outcomes could arise from slightly different neural circuits across sexes, warranting a similar examination of sex differences also in markers of neural activity.
Finally, it is important to recognize that there might be a fundamental difference between the social transmission of emotional information, as measured using the social transmission of distress and freezing in our protocol, for which we find no sex differences, and other phenomena associated with empathy. For instance, prosocial motivation, as measured using directed helping does show sex and familiarity differences in rats. For instance, the latency to liberate a trapped conspecific is shorter in more familiar animals and in females43,51. Also empathy proper, as measured in humans, is stronger in women than men52. Although many assume that emotional contagion is a precursor of empathy and helping, these are clearly not the same phenomena7,53 and that the former but not the latter show sex and familiarity effects suggests that they are subject to different regulatory mechanisms. The exact causal relationship between these phenomena, and how they exchange information and are regulated very remains to be explored, and both male and female animals should be used to explore these systems.
Methods
Subjects
Ten male and nine female Long Evans rats (observers) were bred in house at the animal facility of the Netherlands Institute for Neuroscience. We bred these animals inhouse as part of a larger study exploring early life stress using a limited bedding and nesting manipulation replicatin of54 but we mainly present the data from the control condition here (except for Fig. 4). The breeding led to slightly more males, explaining the slight difference in numbers. Upon weaning animals were housed in same-sex groups of 4, maintained at ambient room temperature (22–24 °C, 55% relative humidity, SPF, type IV cages, on a 12:12 light-dark cycle: lights on at 07:00) till 6 weeks of age. In previous studies, we always ordered demonstrators from Janvier17,27,28. Accordingly, here we also ordered ten male and nine female Long Evans rats (demonstrators; 6 weeks of age) from Janvier Labs (France). Upon arrival, animals were pair-housed with observers (same-sex pairs) in type III cages with wooden block toys, on a reversed 12:12 light-dark cycle (lights off at 07:00). Food and water were provided ad libitum.
Ethics statement
In compliance with Dutch and European law and institutional regulations, all experimental procedures were preapproved by the Centrale Commissie Dierproeven (central committee of animal testing) of the Netherlands (AVD801002015105) and by the welfare body of the Netherlands Institute for Neuroscience (IVD, protocol number NIN161108) in accordance with the Experiments on Animals Act (WOD) with its amendment on 18 December 2014 and EU directive 2010/63/EU. All animals from this experiment were handled at least once a week prior to the experimental start and habituated to the experimental room and setup to reduce unnecessary stress. The welfare of the animals was monitored throughout, and no animals had to be sacrificed because of signs of illness. After experiments, the animals were euthanized by CO2 inhalation, starting with 40% O2 mixed with 60% CO2 until animals were in deep sleep (as checked by the rear reflex response and breathing depth and frequency) and then switched to 100% CO2 for at least 15 min until no breathing or heartbeats were detected.
Setup
All tests were conducted in a two-chamber apparatus (each chamber L: 24 cm x W: 25 m x H: 34 cm, Med Associates, Fairfax, Vermont, United States) as described in ref. 28 Each chamber consisted of transparent Plexiglas walls and stainless-steel grid rods. The compartments were divided by a transparent perforated Plexiglas separation, which allowed animals in both chambers to see, smell, touch and hear each other. For shock pre-exposure and the emotional contagion tests, one of the chambers was electrically connected to a stimulus scrambler (ENV-414S, Med Associates, Fairfax, Vermont, United States). For video recording of the rats’ behaviors, a Basler GigE camera (acA1300–60gm) was mounted on top of the apparatus controlled by Media Recorder (Noldus, the Netherlands).
Experimental procedures
The experimental procedures consisted of habituation, pre-exposure and test phases (Fig. 1). Ten days prior to the emotional contagion test, all animals were handled every other day for 3 min per day. To habituate animals to the testing conditions, animal dyads were transported and placed in the testing apparatus for 20 min per day for three sessions. The testing apparatus was cleaned with lemon-scented dishwashing soap and 70% alcohol between each dyad. To enhance the emotional contagion response to the distress of the demonstrators, observer animals experienced a shock pre-exposure session16,28. The shock pre-exposure was conducted in one of the chambers of the test apparatus. To prevent contextual fear on the test day, the walls of the chamber were coated with black and white striped paper, the background music was turned off, the apparatus was illuminated with bright white light and the chamber was cleaned with rose-scented dishwashing soap and vanilla aroma drops for the pre-exposure session. Observers were individually placed in the apparatus and after a 10 min baseline plus a random interval (~230 s), four footshocks (each: 0.8 mA, 1 s long, 200–260 s random inter-shock interval) were delivered. After the shock pre-exposure session, animals were placed for 1 hour in a neutral cage prior to returning to their home cage. The emotional contagion test setup was illuminated with dim red light, cleaned using a lemon-scented dishwashing soap followed by 70% alcohol, and background radio music was turned on. Observer-demonstrator dyads were transported during their dark-cycle to the testing room and animals were placed in the corresponding chamber of the testing apparatus. For all dyads, following a 12 min baseline, the demonstrators experienced five footshocks (each: 1.5 mA, 1 s long, 120 or 180 s inter-shock interval). Following the last shock, dyads were left in the apparatus for 2 additional min prior to return to their home cage.
Behavior scoring
The behaviors of observers and demonstrators during the emotional contagion test and pre-exposure were manually scored by 2 experienced researchers (inter-rater reliability: Cohen’s Kappa = 0.933) and using the open-source Behavioral Observation Research Interactive Software (BORIS, Friard & Gamba, 2016) as described in ref. 28. Freezing, defined as lack of movement except for breathing movements, was continuously scored throughout the baseline and the shock period. Freezing had to last for at least 2 seconds to be scored as freezing. To create a continuous time series, freezing moments extracted from the Boris result files were recoded as 1 and non-freezing moments as 0 using Matlab (MathWorks inc., USA). We also scored rearing/climbing behaviors (i.e. front paws/both front and rear paws away from the floor and not grooming) using Boris.
Statistics
For sections 2.1 and 2.2, freezing time was calculated as the sum of all freezing moments in a certain epoch and freezing percentage was calculated as the total freezing time divided by the total time of the epoch. Baseline period (1st epoch) was defined as the first 720 seconds of the emotional contagion test and the shock period (2nd epoch) was defined as the 720 seconds following the first shock (approx. 720 s from the start of the test). Statistics were computed using JASP (version 0.11.2, https://jasp-stats.org/). We also include Bayesian statistics in our analysis because a non-significant traditional p-value for a factor (e.g. sex) is not evidence of absence. Bayesian statistics, however, can provide evidence for the absence of an effect if the Bayes factor shows that models without this effect are much more plausible given the data than models with this effect55. Bayesian results are presented as Bayes Factors, with the index specifying whether it is the Bayes Factor in favor of the hypothesized effect H1 or the null hypothesis H0, with BF10 = P(data|H1)/P(data|H0) and BF01 = P(data|H0)/P(data|H1). BF10 > 3 represents moderate evidence for an effect, BF10 < 1/3 for the absence of an effect, and the reverse is true for BF01. BF values around 1 indicate the data is similarly likely under H0 and H1, and cannot adjudicate in favor of either. For model comparisons, BFincl represents the ratio between the likelihood of models including, divided by those excluding a particular factor. BFincl > 3 is interpreted as moderate evidence that the inclusion of this particular factor improves the model. BFincl < 1/3 is interpreted as moderate evidence that this particular factor does not improve the model. Default priors are used throughout. This includes for ANOVAs and ANCOVAs: r = 0.5 for fixed effects (e.g. sex), r = 1 for random effects (e.g. subjects) and r = 0.354 for covariates; and for simple comparisons between groups a Cauchy with a scale of 0.707.
Granger causality
Granger causality is a statistical concept of causality that is based on prediction56. If a signal X1 “Granger-causes” (or “G-causes”) a signal X2, then past values of X1 should contain information that helps predict X2 above and beyond the information contained in past values of X2 alone. In this study, X1 and X2 were binary time series of freezing of the demonstrator and freezing of the observer (freezing coded as 1 and not-freezing coded as 0) on a second-to-second basis. The freezing of the observer at a certain time point (X2(t)) can be estimated either by its own history plus a prediction error (reduced model, 1) or also including the history of the freezing of the demonstrator (full model, 2):
In Eqs. 1 and 2, t indicates the different time points (in steps of 1 s), A represents the regression coefficients and m refers to the model order which is the length of the history included. Granger causality from the freezing of the demonstrator to the freezing of the observer (i.e. X1→X2) is estimated by comparing the full model (2) to the reduced model (1). Mathematically, the log-likelihood of the two models (i.e. G-causality value F) is calculated as the natural logarithm of the ratio of the residual covariance matrices of the two models (3):
This G-causality magnitude has a natural interpretation in terms of information-theoretic bits-per-unit-time57. In this study, for example, G-causality from the demonstrator to the observer indicates the predictive power of the demonstrator’s freezing on the observer’s freezing and the strength of the information flow from the demonstrator to the observer. Jumping responses of the demonstrator to the foot shocks were also taken into account and a binary time series of this behavior was included as X3 (jumping coded as 1 and not-jumping coded as 0).
The algorithms of the Multivariate Granger Causality (MVGC) Toolbox57 in MATLAB were used to estimate the magnitude of the G-causality values. First, the freezing time series of the demonstrators and the observers were smoothed with a Gaussian filter (size = 300 s, sigma = 1.5). The MVGC toolbox confirmed that each time series passed the stationary assumption for Granger causality analysis. Then, the optimal model order (m, the length of history included) was determined by the Akaike information criterion (AIC) for the model including all observer-demonstrator dyads. The optimal model order is a balance between maximizing goodness of fit and minimizing the number of coefficients (length of the time series) being estimated. For this dataset, the model order of 19 (i.e. 19 data points corresponding to 19 seconds in this study) was estimated to be the best fit for the model including all dyads and thus it was fixed at 19 for the subsequent dyad-wise analysis. To test the differences of the G-causality values sexes, we used the G-causality values obtained from analysis only considering the freezing during the shock epoch of a given pair of rats, and compared them across groups using ANOVA in SPSS and JASP.
Data availability
All data (except for the movies from the behavior) can be found at https://doi.org/10.17605/OSF.IO/K6TF7.
Change history
23 July 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Lamm, C., Decety, J. & Singer, T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage 54, 2492–2502 (2011).
Gonzalez-Liencres, C., Shamay-Tsoory, S. G. & Brüne, M. Towards a neuroscience of empathy: Ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci. Biobehav. Rev. 37, 1537–1548 (2013).
Eisenberg, N. & Lennon, R. Sex differences in empathy and related capacities. Psychol. Bull. 94, 100–131 (1983).
Christov-Moore, L. et al. Empathy: Gender effects in brain and behavior. Neurosci. Biobehav. Rev. 46, 604–627 (2014).
Hein, G. & Singer, T. I feel how you feel but not always: the empathic brain and its modulation. Curr. Opin. Neurobiol. 18, 153–158 (2008).
De Waal, F. B. M. & Preston, S. D. Mammalian empathy: Behavioural manifestations and neural basis. Nat. Rev. Neurosci. 18, 498–509 (2017).
Preston, S. D. & de Waal, F. B. M. Empathy: Its ultimate and proximate bases. Behav. Brain Sci. 25, 1–20 (2002).
Panksepp, J. & Panksepp, J. B. Toward a cross-species understanding of empathy. Trends Neurosci. 36, 489–496 (2013).
Keysers, C. & Gazzola, V. A plea for cross-species social neuroscience. in Current Topics in Behavioral. Neurosci. 30, 179–191 (2016).
Lorenz, K. Der Kumpan in der Umwelt des Vogels. J. für Ornithol. 83, 289–413 (1935).
Doherty, R. W., Orimoto, L., Singelis, T. M., Hatfield, E. & Hebb, J. Emotional contagion: Gender and Occupational Differences. Psychol. Women Q. 19, 355–371 (1995).
Magen, E. & Konasewich, P. A. Women support providers are more susceptible than men to emotional contagion following brief supportive interactions. Psychol. Women Q. 35, 611–616 (2011).
Morgan, A. J. & Jorm, A. F. Self-help interventions for depressive disorders and depressive symptoms: A systematic review. Ann. Gen. Psychiatry 7, 14 (2008).
Hoffman, M. L. Sex differences in empathy and related behaviors. Psychol. Bull. 84, 712–22 (1977).
Simner, M. L. Newborn’s response to the cry of another infant. Dev. Psychol. 5, 136–150 (1971).
Atsak, P. et al. Experience modulates vicarious freezing in rats: A model for empathy. PLoS One 6, (2011).
Carrillo, M. et al. Repeated witnessing of conspecifics in pain: Effects on emotional contagion. PLoS One 10, 1–11 (2015).
Meyza, K. & Knapska, E. What can rodents teach us about empathy? Curr. Opin. Psychol. 24, 15–20 (2018).
Meyza, K. Z., Bartal, I. B. A., Monfils, M. H., Panksepp, J. B. & Knapska, E. The roots of empathy: Through the lens of rodent models. Neurosci. Biobehav. Rev. 76, 216–234 (2017).
Pereira, A. G., Cruz, A., Lima, S. Q. & Moita, M. A. Silence resulting from the cessation of movement signals danger. Curr. Biol. 22, R627–R628 (2012).
Jeon, D. et al. Observational fear learning involves affective pain system and Ca v 1.2 Ca 2+ channels in ACC. Nat. Neurosci. 13, 482–488 (2010).
Keum, S. & Shin, H. S. Rodent models for studying empathy. Neurobiol. Learn. Mem. 135, 22–26 (2016).
Langford, D. J., Crager, S. E., Shehzad, Z. & Smith, S. B. Social Modulation of Pain as Evidence for Empathy in Mice. Sci. 312, 1967–1970 (2006).
Gonzalez-Liencres, C., Juckel, G., Tas, C., Friebe, A. & Brüne, M. Emotional contagion in mice: The role of familiarity. Behav. Brain Res. 263, 16–21 (2014).
Keum, S. et al. Variability in empathic fear response among 11 inbred strains of mice. Genes, Brain Behav. 15, 231–242 (2016).
Sanders, J., Mayford, M. & Jeste, D. Empathic Fear Responses in Mice Are Triggered by Recognition of a Shared Experience. PLoS One 8, e74609 (2013).
Carrillo, M. et al. Emotional Mirror Neurons in the Rat’s Anterior Cingulate Cortex. Curr. Biol. 29, 1301–1312.e6 (2019).
Han, Y. et al. Bidirectional cingulate-dependent danger information transfer across rats. PLoS Biol. 17, e3000524 (2019).
Allsop, S. A. et al. Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell 173, 1329–1342.e18 (2018).
Kim, S., Mátyás, F., Lee, S., Acsády, L. & Shin, H. S. Lateralization of observational fear learning at the cortical but not thalamic level in mice. Proc. Natl. Acad. Sci. USA 109, 15497–15501 (2012).
Keum, S. et al. A Missense Variant at the Nrxn3 Locus Enhances Empathy Fear in the Mouse. Neuron 98, 588–601.e5 (2018).
Mogil, J. S. Perspective: Equality need not be painful. Nat. 535, S7 (2016).
Gruene, T. M., Flick, K., Stefano, A., Shea, S. D. & Shansky, R. M. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife 4, (2015).
Fernandes, C., González, M., Wilson, C. & File, S. Factor Analysis Shows That Female Rat Behaviour Is Characterized Primarily by Activity, Male Rats Are Driven by Sex and Anxiety. Pharmacol. Biochem. Behav. 64, 731–736 (1999).
Langford, D. J. et al. Social approach to pain in laboratory mice. Soc. Neurosci. 5, 163–170 (2010).
Langford, D. J. et al. Varying perceived social threat modulates pain behavior in male mice. J. Pain. 12, 125–132 (2011).
Pisansky, M. T., Hanson, L. R., Gottesman, I. I. & Gewirtz, J. C. Oxytocin enhances observational fear in mice. Nat. Commun. 8, 2102 (2017).
Smith, M. L., Hostetler, C. M., Heinricher, M. M. & Ryabinin, A. E. Social transfer of pain in mice. Sci. Adv. 2, e1600855 (2016).
Chen, Q. L., Panksepp, J. B. & Lahvis, G. P. Empathy is moderated by genetic background in mice. PLoS One 4, e4387 (2009).
Rogers-Carter, M. M., Djerdjaj, A., Culp, A. R., Elbaz, J. A. & Christianson, J. P. Familiarity modulates social approach toward stressed conspecifics in female rats. PLoS One 13, e0200971 (2018).
Mikosz, M., Nowak, A., Werka, T. & Knapska, E. Sex differences in social modulation of learning in rats. Sci. Rep. 5, 18114 (2015).
Ishii, A., Kiyokawa, Y., Takeuchi, Y. & Mori, Y. Social buffering ameliorates conditioned fear responses in female rats. Horm. Behav. 81, 53–58 (2016).
Ben-Ami Bartal, I., Decety, J. & Mason, P. Empathy and pro-social behavior in rats. Sci. 334, 1427–30 (2011).
Magrath, R. D., Haff, T. M., Fallow, P. M. & Radford, A. N. Eavesdropping on heterospecific alarm calls: From mechanisms to consequences. Biol. Rev. 90, 560–586 (2015).
Potvin, D. A., Ratnayake, C. P., Radford, A. N. & Magrath, R. D. Birds Learn Socially to Recognize Heterospecific Alarm Calls by Acoustic Association. Curr. Biol. 28, 2632–2637.e4 (2018).
Templeton, C. N. Animal Communication: Learning by Listening about Danger. Curr. Biol. 28, R892–R894 (2018).
Thode, H. C. Testing for normality. 164, (CRC press, 2002).
Jeffreys, H. Theory of Probability. (Oxford University Press, 1961).
Kiyokawa, Y. & Hennessy, M. B. Comparative studies of social buffering: A consideration of approaches, terminology, and pitfalls. Neurosci. Biobehav. Rev. 86, 131–141 (2018).
Takahashi, Y. et al. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav. Brain Res. 240, 46–51 (2013).
Ben-Ami Bartal, I., Rodgers, Da, Bernardez Sarria, M. S., Decety, J. & Mason, P. Pro-social behavior in rats is modulated by social experience. Elife. 3, e01385 (2014).
Ch.ristov-Moore, L. et al. Empath.y: Gender effects in brain and beh.avior. Neuroscience and Biobeh.avioral Reviews 46, 604–627 (2014).
de Waal, F. B. M. Putting th.e Altruism Back into Altruism: Th.e Evolution of Empath.y. Annu. Rev. Psych.ol. 59, 279–300 (2008).
Molet, J., Maras, P. M., Avish.ai-Eliner, S. & Baram, T. Z. Naturalistic rodent models of ch.ronic early-life stress. Developmental Psych.obiology 56, 1675–1688 (2014).
Dienes, Z. Using B ayes to Get th.e Most out of Non-Significant Results. Front. Psych.olology 5, 781 (2014).
Granger, C. W. J. Investigating Causal Relations by Econometric Models and Cross-spectral Meth.ods. Econometrica 37, 424 (1969).
Barnett, L. & Seth., A. K. Th.e MVGC multivariate Granger causality toolbox: A new approach. to Granger-causal inference. J. Neurosci. Meth.ods 223, 50–68 (2014).
J.ourdan, D., Ardid, D., Ch.apuy, E., Esch.alier, A. & Le Bars, D. Audible and ultrasonic vocalization elicited by single electrical nociceptive stimuli to th.e tail in th.e rat. Pain 63, 237–49 (1995).
Sotocinal, S. G. et al. Th.e Rat Grimace Scale: A partially automated meth.od for quantifying pain in th.e laboratory rat via facial expressions. Mol. Pain 7, (2011).
Acknowledgements
We thank Nine Kompier for her help with scoring the freezing behavior of the animals. This work was supported by the Netherlands Organization for Scientific Research (VICI: 453-15-009 to C.K. and VIDI 452-14-015 to VG) and the European Research Council of the European Commission (ERC-StG-312511 to C.K.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Han, Y., Sichterman, B., Carrillo, M. et al. Similar levels of emotional contagion in male and female rats. Sci Rep 10, 2763 (2020). https://doi.org/10.1038/s41598-020-59680-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59680-2
This article is cited by
-
Prosocial behavior, social reward and affective state discrimination in adult male and female mice
Scientific Reports (2023)
-
Audible pain squeaks can mediate emotional contagion across pre-exposed rats with a potential effect of auto-conditioning
Communications Biology (2023)
-
Vicarious Emotions of Fear and Pain in Rodents
Affective Science (2023)
-
Relative to females, male rats are more willing to forego obtaining sucrose reward in order to prevent harm to their cage mate
Psychopharmacology (2023)
-
The role of the anterior insula during targeted helping behavior in male rats
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.