Abstract
Perceptual decision-making on emotions involves gathering sensory information about the affective state of another person and forming a decision on the likelihood of a particular state. These perceptual decisions can be of varying complexity as determined by different contexts. We used functional magnetic resonance imaging and a region of interest approach to investigate the brain activation and functional connectivity behind two forms of perceptual decision-making. More complex unbiased decisions on affective voices recruited an extended bilateral network consisting of the posterior inferior frontal cortex, the orbitofrontal cortex, the amygdala, and voice-sensitive areas in the auditory cortex. Less complex biased decisions on affective voices distinctly recruited the right mid inferior frontal cortex, pointing to a functional distinction in this region following decisional requirements. Furthermore, task-induced neural connectivity revealed stronger connections between these frontal, auditory, and limbic regions during unbiased relative to biased decision-making on affective voices. Together, the data shows that different types of perceptual decision-making on auditory emotions have distinct patterns of activations and functional coupling that follow the decisional strategies and cognitive mechanisms involved during these perceptual decisions.
Similar content being viewed by others
Introduction
Every day we consistently discriminate, identify and evaluate the emotions of others, that is, we make decisions about which emotions we perceive. Decision-making on perceived emotions involves gathering information about the emotional state of another person through the senses, evaluating and integrating that information according to the goals1 and predictions2 of the perceiver, and forming a decision. In contrast to decision-making in general, perceptual decision-making (PDM) emphasizes the role of available sensory information in choosing one option from a set of alternatives3. In experimental paradigms, the perceptual decision-making process is investigated with an additional step in the form of a behavioral response (e.g. a verbal report or a button press).
In the classic framework of information processing, PDM should be the product of a succession of steps from sensory processing to decision formation and, if necessary, motor execution4. However, attempts to fit this division of labor to neural data have encountered several challenges. Functions that should be unified appear distributed throughout the brain5, whereas functions that should be distinct appear to involve the same regions6, or even the same brain cells7,8. Traditionally, extracting sensory information about the world has been shown to take place in specific regions in the brain (e.g. tactile information9; faces10, voices11). The intermediate stage of PDM, i.e. decision formation, is arguably the most enigmatic due to the unsuccessful attempts of researchers to pinpoint specific anatomical regions. A major source of this elusiveness is the varying definition of what should implicate a region in decision formation, and the subsequent methodology used12,13. As a result, there are broad inconsistencies in both the number and the location of brain regions implicated in perceptual decision formation in humans14,15,16,17,18,19,20.
Despite the ongoing debate, the general principle that arises from the literature on PDM is the need for strong bilateral connections between regions of sensory encoding, decision formation and, if required, regions of motor execution. Because there are separate pathways for processing stimuli depending on their perceptual modality and their inherent features (e.g. the ‘what’ and the ‘where’ pathways21,22,23), it stands to reason that the brain networks involved in PDM might also be modulated by these factors. Furthermore, top-down signals from prefrontal cortices to sensory-processing regions ensure that perception remains flexible, in line with the task demands and contextual needs24,25,26. Consequently, instead of a single region or network involved in perceptual choices, likely several networks and mechanisms are involved in deciding what is being perceived. Our brains are factoring in the sensory information at hand as well as contextual cues and prior experience with the stimulus27,28,29, and decide what is being perceived.
There is an impressive amount of information both in the form of empirical data and computational models on how PDM occurs in the visual domain13,30. Significantly less literature exists for the auditory domain, where the decisional process is gradually constrained by serially incoming information. Nevertheless, the same basic principles are assumed in auditory PDM17. For perceiving human voices, in particular, the superior temporal cortex (STC) is a region of sensory encoding11,31 while the inferior frontal cortex (IFC) is likely a region of decision formation32,33,34. The IFC is the receiving end of both dorsal and ventral processing streams with information fed from the STC23. In line with the notion that auditory regions parse the speech signal at different time scales35,36,37, the IFC may have access to several types of temporal acoustic information. The IFC is highly convoluted, cytoarchitectonically diverse and asymmetric not only on the whole38 but also at the level of its sub-regions39,40,41,42. In effect, this makes the IFC a gyral complex. Aside from structure and morphology, its sub-regions are also functionally distinct43,44 yet highly interconnected45. The sub-regions may further serve distinct mental functions, with the left pars opercularis having as many as five separate functional clusters, as revealed by connectivity-based parcellations44.
Specifically concerning decisions on emotional voices, the inferior frontal cortex (IFC) and the orbitofrontal cortex (OFC) have consistently been involved in evaluating perceived vocal emotions36,46. Consequently, several models have placed the IFC and OFC in a crucial role for evaluating affective voices47,48. These structures have close functional and structural connections with the auditory cortex23,49, especially during more cognitively demanding decisions50, and they display functional specialization in processing affective voices at the level of their sub-regions, depending on the attentional focus and the medium of vocal expressions36. To process emotions from the human voice, a temporo-frontal network is believed to mostly operate in a hierarchical manner47,51, including sensory-feature extraction and integrative processes in the superior temporal cortex (STC), as well as evaluative decisions in the IFC/OFC. Additional subcortical regions, such as the amygdala, are recruited at both sensory processing and decision-making levels52,53. Thus, PDM on affective voices consists of a core network of auditory, inferior frontal, and subcortical brain regions54.
The present study used two frequent types of PDM and investigated how the brain adapts to contextual demands in determining what is being perceived. In separate blocks, we asked participants to discriminate identical emotional voices based on two sets of instructions that emphasized the presence or absence of an a priori bias (Fig. 1A). An unbiased decision assumes an equal race between several competing choices, and the final answer is decided in a winner-takes-all fashion after enough information has been serially accumulated55. Such decisions usually appear in contexts where the perceiver has sufficient time to make an informed decision according to several choice options. For decisions with three possible choices, this can be represented as an “A-or-B-or-C” decision task. A biased decision, on the other hand, channels the decisional process towards a particular target (i.e. a specific emotion), and the perceiver tries to confirm or disconfirm the match between a perceptual template of the target answer and the external stimulus56. Regardless of the number of possible choice options, this decision can be represented as an “A-or-not-A” decision, such as selectively monitoring for threat information in a dangerous environment (i.e. “threat” or “no threat”). Biased decisions are a case of conditional expectations, in which the anticipation of one perceptual alternative is favored56. As such, they go beyond a classic two-alternative forced-choice tasks by making the target option (e.g. a threat information) more salient57,58,59, channeling cognitive resources towards the target characteristics and away from unwanted or irrelevant features30,60, and improving speed and accuracy of decisions61.
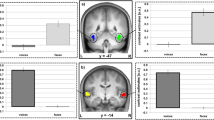
Task instructions and behavioral performance. (A) During unbiased decisions (“A or B or C” decision; upper panel), participants were asked to categorize affective voices as angry, neutral, or happy. During biased decisions (“A or not-A” decision; lower panel), participants were asked to decide whether a perceived voice was either angry or not angry, or happy or not happy. The color points to the presence (red color) or absence (blue color) of an a priori bias towards the choice options. (B) Reaction times and accuracy for biased and unbiased decisions on angry, happy, and neutral voices.
To reveal patterns of brain activation and functional connectivity for biased and unbiased decisions on affective voices, we used functional magnetic resonance imaging (fMRI) and psychophysiological interaction (PPI) analysis. Based on previous literature23,36,49,50,62,63, we focused specifically on the temporo-frontal network and the amygdala. We hypothesized that biased and unbiased decisions would recruit distinct sub-regions within the IFC/OFC, and that unbiased decisions would display stronger functional coupling between frontal, auditory, and limbic regions relative to biased decisions. A further hypothesis was that, if biased and unbiased decisions posed different levels of difficulty to the participants (i.e. maintaining three choice options instead of two choice options in working memory, respectively), then we would expect to see differential practice effects on reaction times and accuracy from the first block to the second for unbiased decisions but not biased decisions.
Results
Behavioral analysis
For all behavioral analyses, condition-specific mean reaction times were computed for each participant. To determine whether there are differential practice effects for biased and unbiased decisions, we included block order as an additional factor in our behavioral analyses. A two (decision: biased and unbiased) by three (emotion: angry, happy, neutral) by two (block order: first and second) repeated-measures ANOVA on reaction times during correct trials revealed a main effect of decision (F1,15 = 6.228, p = 0.025) in which biased decisions were performed faster than unbiased decisions, and a main effect of emotion (F2,30 = 36.828, p < 0.001), but no interaction effect between decision and emotion (F2,30 = 1.398, p = 0.263). Pairwise comparisons (Bonferroni correction) revealed that there were no differences between making decisions on angry versus neutral voices (p = 0.186), whereas happy voices were processed more slowly than both neutral voices (p < 0.001) and angry voices (p < 0.001) (Fig. 1B). In addition, we found no main effect of block order on reaction times (F1,15 = 2.232, p = 0.156), nor an interaction effect between block order and decisional strategy (F1,15 = 0.06, p = 0.940) or block order and emotion (F1,15 = 0.071, p = 0.931) or decision x emotion x order (F2,30 = 0.416, p = 0.663).
The overall accuracy averaged across decisions and participants was very high (96.64%) and a two (decision: biased and unbiased) by three (emotion: angry, happy, neutral) by two (block order: first and second) repeated-measures ANOVA revealed that there were no differences in accuracy between decisions (F1,15 = 1.215, p = 0.288), emotions (F2,30 = 1.036, p = 0.367) or blocks (F1,15 = 0.394, p = 0.539), nor were there any interaction effects between decision and emotion (F2,30 = 0.968, p = 0.391), decision and block order (F1,15 = 1.384, p = 0.258), emotion and block order (F2,30 = 0.813, p = 0.453) (Fig. 1B).
To test whether emotions can differentially modulate the decisional strategy, we looked at the potential behavioral effects of a bias towards a particular auditory emotion (i.e. happy and angry voices, excluding neutral). This analysis did not take block order into account since the previous analysis did not reveal any effect. A three (decision: biased target, biased non-target, unbiased) by two (emotion: happy, angry) repeated-measures ANOVA on reaction times during correct trials revealed a main effect of decision (F 2,14 = 4.05, p = 0.041) and a main effect of emotion (F 1,15 = 55.46, p < 0.001; with angry voices being identified faster than happy voices), but no interaction effect (F 2,14 = 0.24, p = 0.791). Pairwise comparisons (Bonferroni correction) revealed that biased target decisions were performed faster than unbiased decisions (p < 0.030), but there were no differences between biased target and biased non-target decisions (p = 0.278), or between unbiased and biased non-target decisions (p = 0.283).
Functional brain data of the voice localizer scan
The voice localizer scan revealed bilateral activations in several regions in the bilateral STC when human speech and non-speech vocalizations were compared against non-human sounds and vocalizations (Fig. 2A).
Functional activity for the localizer task and the different types of decision-making. (A) Whole-brain activations and contrast estimates for the functional voice localizer scan. (B) ROI brain activations for comparing biased and unbiased decisions on affective voices (i.e. excluding neutral voices). Unbiased decisions on affective voices (red to yellow gradient) activated the right OFC, bilateral STC, amygdala, and bilateral IFCOper extending into the IFCTri, while biased decisions (blue to green gradient) activated the right IFCTri. Dotted black lines mark the voice-sensitive areas as determined by the functional voice localizer scan. (C) Contrast estimates for peak activations in comparisons of biased and unbiased decisions on affective voices. The depicted color bars refer to T-maps. Abbreviations: IFC = inferior frontal cortex; TVA = temporal voice area; human = human speech and non-speech vocalizations; non-human = non-human vocalizations and sounds; IFCOper, IFCTri, IFCOrb = pars opercularis, pars triangularis, and pars orbitalis of the IFC, respectively; OFC = orbito-frontal cortex; STC = superior temporal cortex; Amy = amygdala; Emo = emotional voices (angry and happy); Neu = neutral voices; L = left; R = right.
Functional brain data of the main experiment
Concerning the brain activations in our ROIs underlying the different types of decisions, we computed two different contrasts. First, because we wanted to explore the differences between types of decisions on affect alone, we compared the brain activity for affective voices (i.e. angry and happy voices, excluding neutral) between decisional tasks. This analysis was done because of the different status of the neutral voices during the biased and unbiased decision tasks. We found activations in the bilateral amygdala, bilateral STC, bilateral IFCOper, and right OFC during unbiased decisions ([unbiased decision: affective voices > biased decision: affective voices]), whereas biased decisions on affective voices, compared with unbiased decisions ([biased decision: affective voices > unbiased decision: affective voices]), revealed activations in the right mid IFCTri (Fig. 2B,C, Table 1C,D). Second, for exploratory reasons, we also performed the same contrast, including all types of vocal affect (i.e. anger, happy, or neutral) perceived. Similar to the first analysis, unbiased versus biased decisions elicited activity with peak locations in the bilateral STC, amygdala, IFCOper, and right OFC, while the reversed contrast between biased and unbiased decisions revealed distinct activations in the bilateral IFCTri (Fig. 3, Table 1A,B).
Functional activity in our regions of interest (ROI) analysis for the main effect of decision. The red to yellow gradient refers to unbiased versus biased decisions on all voices (angry, happy, and neutral). The blue gradient refers to biased versus unbiased decisions on all voices (angry, happy, and neutral). Dotted black lines mark the voice-sensitive areas as determined by the functional voice localizer scan. The depicted color bars refer to T-maps. For abbreviations, see Fig. 2.
Regarding the brain activity related to an emotion affect (i.e. processing of affective relative to neutral voices), we did not find significant activation in our ROIs across or within the decisional tasks. However, we found an interaction effect when we compared unbiased with biased decisions ([unbiased decision: affective > neutral voices] > [biased decision: affective > neutral voices]) in the bilateral IFCTri and right IFCOper, as well as in the bilateral voice-sensitive areas in the STC (Fig. 4A,B, Table 1E).
Functional activity for the processing of affective voices. (A) ROI activations pertaining to the interaction effect of decision and emotion ([unbiased decision: affective > neutral voices] > [biased decision: affective > neutral voices]) in the right IFCOper, bilateral IFCTri, and bilateral STC. Dotted black lines mark the voice-sensitive areas as determined by the functional voice localizer scan. (B) Contrast estimates for peak activations for the interaction between emotion and decision ([unbiased decision: affective > neutral voices] > [biased decision: affective > neutral voices]). The solid lines represent functional connections that overlap with connections found for unbiased decisions (see Fig. 4B). The depicted color bars refer to T-maps. For abbreviations, see Fig. 2.
Functional connectivity
Peak activations found in our contrasts of interest served as seed regions in a psycho-physiological interaction (PPI) analysis to determine task-specific functional connectivity between regions. Specifically, we were interested in determining which of our ROIs, namely voice-sensitive areas in the STC, amygdala, OFC, and subregions of the IFC, showed significant increases in functional coupling with our seed regions during one type of decision making on affective voices compared to the other, as well as in relation to the emotional effect.
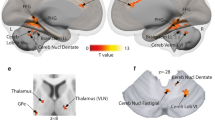
During biased decision-making (excluding neutral voices), no other brain regions significantly coupled with our seed region in the right IFCtri [MNIxyz 58 24 22]. During unbiased decisions on affective voices, compared with biased decisions, bilateral IFCOper was jointly functionally coupled to the bilateral amygdala and the left STC (Figs 5,6A,B, Table 2A,B). Additionally, the right IFCOper [50 8 10] was distinctly connected to the bilateral IFCOrb and the right STC, whereas the left IFCOper [−44 14 4] was distinctly connected to the right OFC. No regions were significantly coupled with the right OFC [32 46 −12], right amygdala [28 −4 −20], or right STC [62 −28 0]. However, peak activations in the left amygdala [−26 −6 −18] and the left STC [−64 −24 6] showed common functional coupling with the left IFCTri (Figs 5, 6C,D, Table 2C,D). Distinct connectivity was shown between the left STC and the left IFCOper, left IFCTri, and bilateral OFC. Additionally, the left amygdala was functionally connected to the right amygdala, right IFCOrb, right STC, and several regions across the left STC during unbiased decisions on affective voices compared to biased decisions.
Functional connectivity during perceptual decision-making. (A) Task-induced functional connectivity during unbiased versus biased decisions on affective voices (i.e. excluding neutral voices). (B) Task-induced functional connectivity during processing of emotional versus neutral voices that is distinct for unbiased decisions. Seed regions and seed connectivity are shown in color. Target regions are shown in grey. The solid lines represent functional connections that overlap with connections found for unbiased decisions. For abbreviations, see Fig. 2.
Task-induced functional connectivity related to the experimental factor decision. Functional connectivity during unbiased versus biased decisions on affective voices (i.e. excluding neutral voices) for seed regions in the (A) IFCOper, (B) right IFCOper, (C) left STC, and (D) left amygdala. (E) Task-induced connectivity related to the interaction between decision (unbiased versus biased) and emotion (affective voices versus neutral voices) with the seed in the right IFCOper. For abbreviations, see Fig. 2.
When making unbiased decisions on affective versus neutral voices, a pattern of functional connectivity emerged that was distinct from making biased decisions on affective versus neutral voices. Specifically, the right IFCOper [38 16 12] was functionally coupled with bilateral amygdala, STC, OFC, IFCTri, and contralateral IFCOper (Figs 5, 6E, Table 2E).
Discussion
In this study, we used fMRI to investigate the brain activation and functional connectivity in two forms of perceptual decision-making on affective voices: biased and unbiased decisions. We found extensive bilateral activation in the posterior IFC (IFCOper) that was unique to unbiased decisions on affective voices. For biased decisions, the only region significantly more active was the right mid IFC (IFCTri), located antero-lateral to the peak activation in the same region during unbiased decision making. This finding suggests a functional distinction within the IFC that reflects the differences in decisional strategies36, possibly linked to the diverse morphology and cytoarchitecture of the IFC64. Such functional differentiation in the IFC has two major implications. First, instead of a single brain region involved in decision formation that adjudicates what is being perceived, there could be distinct decision formation sub-regions within the IFC that respond to contextual demands. This roughly follows proposals of a rostral-to-caudal gradient in the frontal cortex, with more caudal regions being involved in abstract cognitive tasks65. Second, the functional distinction within the IFC could mirror the structural and functional pathways that target the IFC. Specifically, a dual-pathway model posits that a ventral stream from the STC to the more anterior IFC analyzes the global features of the voice while a dorsal stream from the STC to the more posterior IFC analyzes the dynamically varying features37. Global acoustic features, such as mean intensity and pitch, allow gross discrimination of the vocal emotion, on which biased decisions could be based66, whereas the unbiased decision could predominantly rely on varying finer-tuned features23. The need for biased and unbiased decisions to depend on different pathways and IFC sub-regions might come from how affective information is processed from voices. For instance, a one-step classifier of affective voices was found to be inferior to a two-step classifier consisting of a first stage for gross feature extraction, allowing the initial classification of the emotion in one of several broad categories, and a second stage for finer feature extraction and conclusive affective identification67. Perhaps global acoustic features are sufficient for the cursory processing of affective voices and their subsequent biased discrimination, whereas the finer level of unbiased decisions depends on both locally varying and global prosodic cues, thus necessitating stronger coupling between the different brain regions able to extract, process, and represent the affective values of auditory stimuli.
We additionally found activity in the right OFC as well as bilateral amygdala and STC for unbiased versus biased decisions. The amygdala plays an essential role in the accurate processing of the affective meaning of voices52, while the STC provides proper acoustic analysis of relevant voice features and their integration into an auditory percept32. The posterior IFC (IFCOper) might then incorporate this additional affective and acoustic information on the way to a conclusive decision about the emotion that has been heard. Unbiased decisions might be further supported by the OFC, which seems to be involved in the late evaluative stages rather than the early sensory stages of emotional speech processing68.
Biased compared to unbiased decisions increased brain activity only in one region, namely the right IFCTri. We note that this contrast revealed negative beta parameters for both types of decisions. In other words, there was less deactivation in the right IFCtri during biased decisions compared to unbiased decisions. Such pattern of brain activity is not uncommon in neuroimaging69. However, the exact interpretation of this phenomenon is still debated, as it can equally reflect neural inhibition of task-irrelevant neural processing or hemodynamic compensatory mechanisms70. If we consider that the right mid IFC is involved in general behavioral and cognitive inhibition43,71,72, we can speculate that the IFC could also potentially contribute to unbiased decisions on affective voices by a markedly reduced inhibitory activity, in addition to the excitatory input to the amygdala and voice-sensitive areas in the STG that we see in our results.
The absence of significant differences in the BOLD activity of the STC and amygdala for the comparison between biased and unbiased decisions might suggest that biased decisions are largely accomplished without in-depth acoustic and emotional analysis of the voices. If we conceive of the general decision-making process as a “random walk” in which sensory information in favor of one alternative or another is gradually accumulated toward a decision threshold73,74, then we can consider two explanations for our imaging and behavioral results. First, the anticipation of the favored perceptual alternative (i.e. the target emotional voice) during biased decisions might set the origin of the random walk closer towards the threshold for biased choice options12,30,75. Under this account, less bottom-up sensory information needs to be accumulated for accurate perception and for the decision threshold to be reached. Conversely, the diffusion processing for unbiased decisions begins equidistant from the decisional thresholds for the three choice options, thus requiring on average more information to be accumulated to confirm a percept, which then triggers extensive processing in auditory and limbic regions. Second, it is possible that the rate of the acquisition of sensory information is actively modulated by prior expectations30,76. Specifically, the decisional bias increases the salience of the target stimuli, leading to their disproportionally faster processing in the first stages of evidence accumulation, and significantly slower processing in the later stages. Such non-linear mechanism of information accumulation has been proven in sequentially presented evidence of highly salient stimuli77,78,79, similar to the biased emotional voices in our study. De Lange et al.77 found that the brain activity in regions of evidence accumulation was inversely related to the amount of accumulated evidence, such that when more evidence had been accumulated during initial stages of perception, neural activity was attenuated for later stages of perception. It is possible that the restricted pattern of neural activity and connectivity in our results during biased decisions comes also from the limited temporal resolution of the fMRI that can only capture the linear mechanism of evidence accumulation during unbiased decisions. Future studies using more sensitive techniques such as magnetoencephalography77 can provide the much needed temporal resolution for the investigation of biased and unbiased decisions.
Together, the data above highlight the notion that unbiased decisions rely on a distributed neural network that provides relevant information for a proper decision, while biased decisions mostly rely on functional mechanisms provided by a single frontal region. In terms of functional brain connectivity, we did not find significant increased coupling between our ROIs during biased compared with unbiased decisions on affective voices. Instead, we found that the IFC, amygdala, and STC were significantly coupled during unbiased decisions on affective voices compared to biased decisions, thus pointing to an extended neural network rather than a single region underlying these decisions. This connectivity network revealed three notable features. First, bilateral IFCOper was primarily a source of connectivity towards our ROIs, which might then drive co-activation in other regions. Second, the left amygdala and STC were both targets and sources of functional connectivity, while the right amygdala and STC were solely target regions. Third, there appears to be a functional connectivity loop in the left hemisphere between the IFCOper, STC, and amygdala, and this loop also involves the OFC. Concerning the functional meaning of this network, we propose that the IFC detects task difficulty17 and consequently initiates or enhances acoustic and emotional processing in the STC and amygdala, respectively, via top-down influences80. Moreover, this enhanced processing is accompanied by a series of functional cross-talks between the IFC, STC and the amygdala. The STC might interact with the left amygdala in order to incorporate affective information into an integrated percept of the affective voice81, and this emotional gestalt is made available to higher order frontal regions, such as the IFC82 and the OFC83, which support the proper evaluation of the affective meaning of voices.
We additionally found activity in bilateral IFC and STC in the interaction between decision and emotion only for unbiased versus biased decisions. Therefore, the affective decoding of voices in the STC and IFC36,84 becomes relevant only when one is confronted with more complex cognitive mechanisms of decisions that require proper affective classification when given several choice options. The absence of an emotion effect during biased decision-making suggests that the success of this type of decision depends on less acoustic and emotional information compared to the more complex unbiased decisions, in line with the drift-diffusion model61. For example, happy voices could be discriminated from other affective voices by taking into account only simple acoustic features such as fast onsets and tone attacks of the vocalization85 or spectral variability86 without having to analyze the acoustical features related to emotion at a more fine-grained level.
Interestingly, the original contrast for the interaction between emotion and decision did not reveal activity in the amygdala, but the functional connectivity analysis revealed significant coupling of the amygdala with the posterior IFC as the source. This indicates that the affective decoding in the amygdala does not occur instantaneously when confronted with an auditory emotion but rather seems to be triggered by the IFC when required by the complexity of the decisional task80. The affective decoding during more complex decisions therefore depends on a widespread functional network that provides emotional (amygdala) and acoustic information (STC), as well as evaluative processing (IFC and OFC). This pattern of connectivity for the interaction between decision and affect appears to partly overlap with the connectivity pattern between decisions on affective voices. The overlap specifically concerns the right IFCOper as a source of connectivity to the bilateral amygdala and bilateral STC, indicating that during unbiased decisions, these connections initiate the processing of the affective meaning of voices, furthering functional loops in the left hemisphere as outlined earlier.
Strengths and limitations
Because unbiased and biased decisions point to different strategies commonly used to identify stimuli in the environment – in our case, different emotional voices, we wanted to keep their characteristics intact and not artificially control for task differences more than we needed to. We operationalized these decisional strategies as “A or B or C” and “A or not A (i.e. B or C)”, respectively. We called unbiased decisions as such in order to contrast them against biased decisions. A prototypical unbiased decision would involve the free choice of answers in the absence of any predefined categories. Such prototypical decisions exist in behavioral research87 but in the present study the paradigm has been adapted to the fMRI environment where only a button box with a limited number of choices could be used, whereas a verbal output would likely have introduced motion artefacts. Research has shown comparable levels of accuracy between free-choice and fixed-choice identification of emotions87,88.
While it might seem that the two decisions fundamentally differ in the number of choice categories (i.e. three versus two), we argue that the biased decision is not a traditional two-alternative forced-choice task despite the presence of only two possible button presses59. Participants do not perceive two types of stimuli as the “not A” category is not a stimulus class in itself but rather an ad hoc construct. To successfully perform the task, the participants must first identify the stimulus as pertaining to its original class (i.e. angry, happy, neutral voices) and subsequently allocate it to “target” or “non-target”.
One can argue that biased and unbiased decisions differ on too many dimensions and therefore involve different levels of complexity. If that were true, then we would expect to see behavioral and neural correlates of these differences. Task complexity can refer to a multitude of things such as cognitive mechanisms, task difficulty or motor output. Although reaction times were slower for biased compared to unbiased decisions, accuracy rates would be a better indicator for task difficulty and the two decisions did not differ in this regard. This suggests that the two tasks diverge not in difficulty but rather in the underlying computational mechanisms or motor preparation times. Moreover, participants signaled neutral voices with a double button press during unbiased trials and with a single button press during biased trials. Although we mainly focused our analyses on emotional trials only (i.e. angry and happy voices), the different motor scheme during neutral trials could have induced effects pertaining to motor preparation times that spilled over the rest of the trials. Alternatively, it can be argued that the requirement of simultaneously pressing two buttons artificially increased the salience of unbiased trials compared to biased trials. However, when comparing biased and unbiased decisions on neutral voices trials (i.e. single versus double button presses), we did not find significant differences in the neural correlates. Furthermore, we found no main effect of this motor scheme on reaction times (F1,15 = 0.329, p = 0.574) or accuracy (F1,15 = 0.303, p = 0.590) during neutral trials. In light of this, it appears that we have a double dissociation: biased and unbiased decisions showed no differences in the neural correlates nor in reaction times during neutral trials where the motor output diverges the most, but they show significant differences during emotion trials where the motor output is identical. Therefore, the different motor output of the two tasks did not significantly alter our findings. Together with the similar accuracy rates that indicate comparable levels of difficulty, we propose that biased and unbiased decisions should differ in the underlying computational mechanisms. Specifically, biased decisions rely on a gross extraction of features, which allows for the quick identification of the voice, a process that is superficial and yet sufficient for the subsequent classification of the stimulus into a broader category, i.e. target or non-target. Conversely, unbiased decisions require a more extended functional network, which provides in-depth acoustic and emotional information.
During biased and unbiased trials, participants listened to identical stimuli, the varying element being the decisional strategy that is enforced through instructions. In this regard, we believe that the number of choice categories are paramount to ensure the conceptual distinction between the tasks and, therefore, should not be controlled for. Summerfield and Koechlin56 introduced the same decisional bias as we do but in a non-emotional visual task while controlling for the number of buttons. Specifically, their participants had to decide between two Gabor patches using an “A or not-A” decisional strategy (i.e. same as ours) or using an “A or B” strategy. While we appreciate their desire to control for the number of buttons across decisions, we also strongly believe that such paradigm cannot account for the individual strategies that each participant uses. As such, there is no way of determining whether the participant truly used the designated decisional strategy within a particular trial or whether they interchangeably used an “A or not A” or an “A or B” across trials. Having the same number of choices makes it possible for the participant to use the opposite strategy, either intentionally (e.g. personal preference to ensure speed or accuracy) or unintentionally (e.g. a carryover effect introduced by the randomization process). In fact, the authors56 report virtually identical reaction times across “A not A” and “A or B” decisions. Contrary to their approach, we introduce a third choice-category in order to make it more difficult for the participant to use a different strategy other than the designated one and, as discussed above, such manipulation did not introduce unwanted behavioral and neural results.
In conclusion, our study shows that the presence or absence of priming during perceptual decision-making on vocal emotions induces different patterns of functional activity and connectivity in the IFC and OFC. This has direct implications for the research into perceptual decision-making that so far has mainly focused on two-alternative forced-choice tasks3, ignoring the ecological variety of perceptual choices that we make on a constant basis. Different circumstances require different perceptual choices and our results show that the brain adapts to these contextual demands. Scientific inquiry should therefore acknowledge the diversity of perceptual decision-making when interpreting and extrapolating results from specific paradigms. Because of the nature of our stimuli and experimental design, we cannot make any claims as to whether the pattern of functional activity and connectivity is unique to the auditory processing of emotions. Future studies should incorporate different types of modalities of varying degrees of perceptual complexity.
Methods
In the present study, we introduced two types of perceptual decision-making. Biased and unbiased decisions are two strategies with high ecological validity used to identify stimuli in the environment – in our case, vocal emotions. We note that the term bias is used in two ways in the computational neuroscience literature. First, from the viewpoint of sensory information, bias refers to the role that context and prior information have on perception and perceptual choices. Specifically, perception is seen as an inferential process, such that incoming sensory information is not analyzed de novo but interpreted based on prior information24. Second, from the viewpoint of the decisional strategy, the terms biased and unbiased decisions to refer to prior expectations regarding some of the choice categories61,89. During biased decisions, in contrast to unbiased, the participant is asked to focus on a specific emotion category, discriminating each incoming stimulus into either target or non-target. The current study aimed at investigating biased decisions on vocal emotions from the second perspective as this is a prevalent ecological perspective in decision-making on emotions. Furthermore, we call unbiased decisions as such in order to contrast them against biased decisions. A prototypical unbiased decision would involve the free choice of answers in the absence of any predefined categories. Such a paradigm exists in behavioral research but in this study, it has been adapted to the fMRI environment.
Participants
Sixteen healthy volunteers (five males; mean age 24.81 years, SD = 4.41, age range 19–34) took part in the experiment. The participants had normal hearing abilities and normal or corrected-to-normal vision. No participant presented a neurological or psychiatric history. All participants gave informed and written consent for their participation. The local ethics committee of the University of Geneva approved all experimental protocols and methods of data collection, data handling and analysis. All methods and experimental protocols were performed in accordance with the guidelines and regulations of the local ethics committee of the University of Geneva.
Stimuli
The stimulus material consisted of speech-like but semantically meaningless two-syllable words from the Geneva Multimodal Emotion Portrayal (GEMEP) database90,91. These pseudowords were 16-bit recordings sampled at a 44.1 kHz sampling rate and with a mean duration of 675 ms (SD = 192ms). During the experiment, stimuli were presented at 70 dB SPL. One male and one female speaker spoke six different pseudo-words (“belam”, “lagod”, “namil”, “nodag”, “nolan” and “minad”) in an angry, happy, or neutral tone. These twelve pseudo-words (six per gender) were selected from a behavioral evaluation of sixteen pseudo-words (eight per gender) out of the GEMEP database on six emotion scales (arousal, sadness, joy, anger, fear, neutral) performed by 12 participants (9 females, mean age 27.17 years, SD 4.39 years). We selected those pseudo-words that were most consistently evaluated as angry (F 1.30,14.25 = 481.70, p < 0.001, Greenhouse-Geisser (GG) corrected), happy (F 1.43,15.76 = 146.46, p < 0.001, GG corrected), and neutral (F 1.33,14.61 = 447.29, p < 0.001, GG corrected), respectively. One-way repeated-measures ANOVA revealed that there was a statistically significant difference in perceived arousal scores among the angry, happy, and neutral voices (F 2,22 = 202.91, p < 0.001). Pairwise comparisons revealed that arousal scores for angry and happy tones were significantly higher than scores received by neutral tones (p < 0.001), but they did not differ from each other (p = 0.351).
To localize human voice-sensitive regions in the bilateral STC, we presented in a separate run (i.e. referred to as a voice localizer scan) 500 ms sound clips consisting of 70 human speech and non-speech vocalizations, and 70 non-human vocalizations and sounds (animal vocalizations, artificial sounds, natural sounds) presented at 70 dB SPL11.
Experimental design
For the fMRI experiment, we used an event-related design with two runs of unbiased decisions (i.e. three-alternative forced-choice decision of whether the voice was angry, happy, or neutral) and two runs of biased decisions (i.e. decision about the presence or absence of a target emotion). For example, in the two biased decision runs, the same participants received a “happy or not happy” set of instructions and an “angry or not angry” set instructions, respectively. For unbiased decisions, the participants received a “happy or angry” and an “angry or happy” set instructions, respectively. In addition, we counterbalanced these four runs using a Latin Square method, such that each type of instruction was always followed by another type an equal number of times. Within each run, 36 voices (12 per emotional tone) were presented twice inside distinct trials in a partially randomized order, so that the same type of emotion was never presented more than three times in a sequence. Each trial started with a visual fixation cross (1×1°) presented on a black background, followed by the presentation of a voice and then by a jittered blank screen until the onset of the next trial. The jitter for the fixation cross was randomly chosen an equal amount of times from an array of six possible durations (800 ms, 840 ms, 880 ms, 920 ms, 960 ms and 1000 ms). The jitter for the blank screen following the stimulus was randomly chosen an equal number of times from an array of six possible durations (3000 ms, 3400 ms, 3800 ms, 4200 ms, 4600 ms and 5000 ms). The fixation cross prompted the participant’s attention and remained on the screen for the duration of the auditory stimulus. The total length of each run was approximately seven minutes. The stimuli were presented binaurally through MRI-compatible headphones. The participants listened to each voice and made a corresponding button press as soon as they could identify the emotional tone (Fig. 1A). During unbiased decision blocks, participants used both index fingers to identify the emotion heard, as follows: one button press for happy voices, one button press for angry voices, and simultaneous button presses for neutral voices. During biased decision trials, participants pressed one button for the target emotional tone (either happy or angry) and another for the non-target emotional tone (neutral was always a non-target sound). Task blocks alternated across the experiment, and block order and response buttons were counterbalanced across participants.
For the voice localizer, each sound clip was preceded by a 500 ms fixation cross and followed by a jittered 3550–5000 ms (from a uniform distribution in 50 ms steps) gap before the onset of the next stimulus. To maintain participants’ attention, 10 randomly chosen sounds were repeated twice in a row, and participants had to detect and indicate each repetition by an index finger button press.
Image acquisition
We recorded functional imaging data on a 3-T SIEMENS Trio System (Siemens, Erlangen, Germany) using a T2*-weighted gradient multiplexed echo-planar imaging (M-EPI) pulse sequence92 with an acceleration factor of four, 3 mm isotropic resolution, 36 slices in a 64×64 matrix, 20% distance factor, TR/TE = 650/30 ms, and FA = 50°. Structural images had 1 mm isotropic resolution (192 contiguous 1 mm slices, TR/TE/TI = 1900/2.27/900 ms, FoV = 296 mm, in-plane resolution of 1×1 mm). For the voice localizer scan, we used a partial volume acquisition of 28 slices (TR/TE = 650/30ms, thickness/gap = 2/0.4 mm, FoV = 192 mm, in-plane resolution 2×2 mm, FA = 54°) aligned obliquely to the AC-PC plane to cover all portions of the STC from anterior to posterior. Additional physiological data (heart rate and respiration) were recorded with the MP150 Biopac Systems acquisition system (Santa Barbara, CA).
Data analysis
Preprocessing and statistical analyses of functional images were performed with Statistical Parametric Mapping software (SPM8, Welcome Department of Cognitive Neurology, London; www. fil.ion.ucl.ac.uk/spm). Functional data were first manually realigned to the AC-PC axis, followed by motion correction of the functional images. Each participant’s structural image was coregistered to the mean functional image, and then segmented to allow estimation of normalization parameters with the DARTEL algorithm93. Using the resulting flow fields, the anatomical and functional images were spatially normalized to the Montreal Neurological Institute (MNI) stereotactic space. The functional images for the main experiment were resampled into 2 mm3 voxels. All functional images were spatially smoothed with a 6 mm full-width half-maximum (FWHM) isotropic Gaussian kernel.
For the first-level analysis of the voice localizer, all trials were modeled with a stick function aligned to the onset of each stimulus, which was then convolved with a standard hemodynamic response function (HRF). Simple contrasts for vocal and non-vocal stimuli were then taken to a random-effects group-level analysis to determine voice-sensitive regions along the STC in both hemispheres.
For the first-level statistical analyses of our main experiment, we similarly used a general linear model in which each trial was modeled with a stick function set at the onset of the auditory stimuli and in which trial-by-trial reaction times were used as a parametric regressor (see Supplementary Fig. S1). By default, SPM8 performs orthogonalization according to the previous regressor. The stick functions were then convolved with a canonical HRF. Because neutral voices were always non-target in discrimination trials, we treated them as a single condition, regardless of whether the target emotion was angry or happy prosody. This yielded eight experimental conditions (see Supplementary Fig. S2), three for unbiased decisions (angry, happy, or neutral voices) and five for biased decisions, as follows: decisions on angry voices when the bias was towards angry voices (i.e. angry target) or towards happy voices (i.e. angry non-target), decisions on happy voices when the bias was towards happy voices (i.e. happy target) or towards angry voices (i.e. happy non-target), and discrimination of neutral voices (i.e. neutral non-target). For each of the eight experimental conditions, we created a regressor for the correct trials of each type of emotion and one additional regressor for all the incorrect trials collapsed across emotions. Accuracy of the classification of neutral voices was defined as synchronous button presses, regardless of which button was pressed first. Consequently, if only one button was pressed, or no presses were made, the respective neutral trials were labeled as incorrect. Reaction times for classification of neutral voices were calculated based on which of the two buttons were pressed first. Additionally, six motion correction parameters and four physiological parameters were included as regressors of no interest to account for signal changes not related to the conditions of interest. Physiological parameters were analyzed by using Retrospective Image Correction94, as implemented for MATLAB (http://cbi.nyu.edu/software). Simple contrasts for each experimental condition and for each participant were then taken to a random-effects group analysis by using a flexible factorial ANOVA, following the guidelines of Jan Gläscher and Darren Gitelman95. Specifically, for «Factors», we used subject as Factor 1 (Independence set to Yes and Variance set to Equal) and condition as Factor 2 (Independence set to No and Variance set to Equal). For «Subjects», we provided all images for each subject based on a matrix that maps a subject’s image onto the different factor levels. Under «Main effects & Interactions», we included a main effect for Factor 1 (16 levels) and a main effect for Factor 2 (8 levels), and no interactions. In the resulting second-level design matrix (see Supplementary Fig. S2), we looked at the zero-sum linear combination of the condition parameters of interest. All group results were thresholded at a combined voxel threshold of p < 0.05 (FDR corrected) and a cluster extent threshold of k = 10.
Our main focus was to compare biased with unbiased decisions on emotions. As a result, our design was unbalanced, with neutral voices only being non-target during biased discrimination blocks. Such an unbalanced design resulted from needing neutral voices as a third category in order to make the conceptual distinction between biased and unbiased decisions, while aiming to keep the scanning time to a minimum. Consequently, we first explored the main effects of task by comparing functional activations during unbiased decisions on all voices relative to biased decisions, irrespective of the type of emotion. In a second analysis, we contrasted the two types of decisions on affective voices directly (i.e. excluding neutral voices). Additionally, we contrasted affective voices with neutral voices within and across decisional tasks, and we examined the interaction between affective voices and the type of decision in order to reveal emotion effects that are specific for each type of decision making.
Because of our a priori interest in the IFC, the OFC, STC, and amygdala, we restricted our analyses to these predefined regions of interest (ROIs) by applying masks on whole-brain results. We opted for this approach because it is more conservative than small-volume correction. We used the Automated Anatomical Labeling (AAL) atlas96 to define ROIs for the bilateral IFC, OFC, and amygdala. The three bilateral subdivisions of the IFC, namely pars opercularis (IFCOper), pars triangularis (IFCTri), and pars orbitalis (IFCOrb), were combined with the bilateral OFC into a single mask of bilateral IFC-OFC. For the bilateral STC, we created functional ROIs of voice-sensitive auditory cortex identified from the voice localizer scan.
To reveal functional connectivity between our ROIs, we performed a psycho-physiological interaction (PPI) analysis97. For each of our group-level contrasts of interest, peak activations found in bilateral IFC, OFC, STC, and amygdala were chosen as seed regions, from which we extracted the first eigenvariate in a 3 mm radius sphere at the level of each participant. For each seed region, the PPI analysis was set up as a general linear model that included three regressors. The first regressor contained the extracted and deconvolved time course of functional activation within a seed region in each of the four functional runs (the physiological variable). The second regressor included the comparison between unbiased and biased decisions on affective voices (the psychological variable). In other words, we created a time course regressor for the task, including as many sampling points as for the physiological variable. The values in the second regressor were set to “1” for trials of biased decisions on angry and happy voices (excluding neutral) and “−1” for trials of unbiased decisions on angry and happy voices (excluding neutral). Only trials were included in the PPI analysis where participants gave a correct response. A separate analysis included a regressor for trials of unbiased decisions set to “1” and trials of biased decisions set to “−1” (please see Supplementary Fig. S3). Finally, the third regressor included the interaction between the first two regressors, which was created by a point-by-point multiplication of the time course for the physiological variable and the time course for the psychological variable. This interaction regressor was the only regressor of interest, whereas the physiological variable and the psychological variable served as co-variates of no interest in each PPI analysis. Individual results for these PPI analyses for each group seed region were entered into separate second-level flexible factorial analyses, where target regions of functional connectivity were limited to our ROIs. All resulting statistical maps were thresholded at a combined voxel threshold of p < 0.05 (FDR corrected) and a cluster extent threshold of k = 10.
Data availability
Under the Swiss guidelines of data protection (Ordinance HFV Art. 5), the datasets generated and analyzed during the current study can be made available from the corresponding author on a case by case basis.
References
Rangel, A., Camerer, C. & Montague, P. R. A framework for studying the neurobiology of value-based decision making. Nature Reviews Neuroscience 9, 545–556 (2008).
Summerfield, C. et al. Predictive codes for forthcoming perception in the frontal cortex. Science 314, 1311–1314 (2006).
Hauser, C. K. & Salinas, E. Perceptual Decision Making (2014).
Cisek, P. & Kalaska, J. F. Neural mechanisms for interacting with a world full of action choices. Annual review of neuroscience 33, 269–298 (2010).
Spreng, R. N. & Grady, C. L. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. Journal of cognitive neuroscience 22, 1112–1123 (2010).
Mulder, M., Van Maanen, L. & Forstmann, B. Perceptual decision neurosciences–a model-based review. Neuroscience 277, 872–884 (2014).
Gallese, V. & Goldman, A. Mirror neurons and the simulation theory of mind-reading. Trends in cognitive sciences 2, 493–501 (1998).
Gold, J. I. & Shadlen, M. N. The neural basis of decision making. Annu. Rev. Neurosci. 30, 535–574 (2007).
Preuschhof, C., Heekeren, H. R., Taskin, B., Schubert, T. & Villringer, A. Neural correlates of vibrotactile working memory in the human brain. The Journal of neuroscience 26, 13231–13239 (2006).
Kanwisher, N., McDermott, J. & Chun, M. M. The fusiform face area: a module in human extrastriate cortex specialized for face perception. The Journal of Neuroscience 17, 4302–4311 (1997).
Belin, P., Zatorre, R. J., Lafaille, P., Ahad, P. & Pike, B. Voice-selective areas in human auditory cortex. Nature 403, 309–312 (2000).
Dutilh, G. & Rieskamp, J. Comparing perceptual and preferential decision making. Psychonomic bulletin & review, 1–15 (2015).
Kelly, S. P. & O’Connell, R. G. The neural processes underlying perceptual decision making in humans: Recent progress and future directions. Journal of Physiology-Paris 109, 27–37 (2015).
Liu, T. & Pleskac, T. J. Neural correlates of evidence accumulation in a perceptual decision task. Journal of neurophysiology 106, 2383–2398 (2011).
Noppeney, U., Ostwald, D. & Werner, S. Perceptual decisions formed by accumulation of audiovisual evidence in prefrontal cortex. The Journal of Neuroscience 30, 7434–7446 (2010).
Filimon, F., Philiastides, M. G., Nelson, J. D., Kloosterman, N. A. & Heekeren, H. R. How embodied is perceptual decision making? Evidence for separate processing of perceptual and motor decisions. The Journal of Neuroscience 33, 2121–2136 (2013).
Heekeren, H. R., Marrett, S. & Ungerleider, L. G. The neural systems that mediate human perceptual decision making. Nature reviews neuroscience 9, 467–479 (2008).
Tosoni, A., Galati, G., Romani, G. L. & Corbetta, M. Sensory-motor mechanisms in human parietal cortex underlie arbitrary visual decisions. Nature neuroscience 11, 1446–1453 (2008).
Kayser, A. S., Buchsbaum, B. R., Erickson, D. T. & D’Esposito, M. The functional anatomy of a perceptual decision in the human brain. Journal of Neurophysiology 103, 1179–1194 (2010).
Ho, T. C., Brown, S. & Serences, J. T. Domain general mechanisms of perceptual decision making in human cortex. The Journal of Neuroscience 29, 8675–8687 (2009).
Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends in neurosciences 15, 20–25 (1992).
Tsunada, J., Liu, A. S., Gold, J. I. & Cohen, Y. E. Causal contribution of primate auditory cortex to auditory perceptual decision-making. Nature neuroscience 19, 135–142 (2016).
Fruhholz, S., Gschwind, M. & Grandjean, D. Bilateral dorsal and ventral fiber pathways for the processing of affective prosody identified by probabilistic fiber tracking. Neuroimage 109, 27–34, https://doi.org/10.1016/j.neuroimage.2015.01.016 (2015).
Wiech, K. et al. Influence of prior information on pain involves biased perceptual decision-making. Current Biology 24, R679–R681 (2014).
Siegel, M., Engel, A. K. & Donner, T. H. Cortical network dynamics of perceptual decision-making in the human brain. Frontiers in human neuroscience 5, 21 (2011).
Jiang, J., Summerfield, C. & Egner, T. Attention sharpens the distinction between expected and unexpected percepts in the visual brain. The Journal of Neuroscience 33, 18438–18447 (2013).
Reckless, G. E. et al. The left inferior frontal gyrus is involved in adjusting response bias during a perceptual decision‐making task. Brain and behavior 4, 398–407 (2014).
Rahnev, D., Lau, H. & de Lange, F. P. Prior expectation modulates the interaction between sensory and prefrontal regions in the human brain. The Journal of Neuroscience 31, 10741–10748 (2011).
Hanks, T. D., Mazurek, M. E., Kiani, R., Hopp, E. & Shadlen, M. N. Elapsed decision time affects the weighting of prior probability in a perceptual decision task. The Journal of Neuroscience 31, 6339–6352 (2011).
Summerfield, C. & De Lange, F. P. Expectation in perceptual decision making: neural and computational mechanisms. Nature Reviews Neuroscience 15, 745–756 (2014).
Pernet, C. R. et al. The human voice areas: Spatial organization and inter-individual variability in temporal and extra-temporal cortices. NeuroImage 119, 164–174 (2015).
Brück, C., Kreifelts, B., Kaza, E., Lotze, M. & Wildgruber, D. Impact of personality on the cerebral processing of emotional prosody. Neuroimage 58, 259–268 (2011).
Kaiser, J., Lennert, T. & Lutzenberger, W. Dynamics of oscillatory activity during auditory decision making. Cerebral cortex 17, 2258–2267 (2007).
Binder, J. R., Liebenthal, E., Possing, E. T., Medler, D. A. & Ward, B. D. Neural correlates of sensory and decision processes in auditory object identification. Nature neuroscience 7, 295–301 (2004).
Poeppel, D. The analysis of speech in different temporal integration windows: cerebral lateralization as ‘asymmetric sampling in time’. Speech communication 41, 245–255 (2003).
Frühholz, S. & Grandjean, D. Processing of emotional vocalizations in bilateral inferior frontal cortex. Neuroscience & Biobehavioral Reviews 37, 2847–2855 (2013).
Rauschecker, J. P. Ventral and dorsal streams in the evolution of speech and language. Frontiers in Evolutionary Neuroscience 4, 1–4 (2012).
Hammers, A. et al. Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Human brain mapping 28, 34–48 (2007).
Foundas, A. L., Eure, K. F., Luevano, L. F. & Weinberger, D. R. MRI asymmetries of Broca’s area: the pars triangularis and pars opercularis. Brain and language 64, 282–296 (1998).
Foundas, A. L., Weisberg, A., Browning, C. A. & Weinberger, D. R. Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. Journal of Neuroimaging 11, 153–159 (2001).
Yamasaki, S. et al. Reduced gray matter volume of pars opercularis is associated with impaired social communication in high-functioning autism spectrum disorders. Biological psychiatry 68, 1141–1147 (2010).
Tomaiuolo, F. et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. European Journal of Neuroscience 11, 3033–3046 (1999).
Cai, W. & Leung, H.-C. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PloS one 6, e20840 (2011).
Clos, M., Amunts, K., Laird, A. R., Fox, P. T. & Eickhoff, S. B. Tackling the multifunctional nature of Broca’s region meta-analytically: co-activation-based parcellation of area 44. Neuroimage 83, 174–188 (2013).
Greenlee, J. D. et al. Functional connections within the human inferior frontal gyrus. Journal of Comparative Neurology 503, 550–559 (2007).
Dricu, M. & Frühholz, S. Perceiving emotional expressions in others: Activation likelihood estimation meta-analyses of explicit evaluation, passive perception and incidental perception of emotions. Neuroscience & Biobehavioral Reviews 71, 810–828 (2016).
Schirmer, A. & Kotz, S. A. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends in cognitive sciences 10, 24–30 (2006).
Wildgruber, D., Ackermann, H., Kreifelts, B. & Ethofer, T. Cerebral processing of linguistic and emotional prosody: fMRI studies. Progress in brain research 156, 249–268 (2006).
Ethofer, T. et al. Cerebral pathways in processing of affective prosody: a dynamic causal modeling study. Neuroimage 30, 580–587 (2006).
Leitman, D. I. et al. “It’s Not What You Say, But How You Say it”: A Reciprocal Temporo-frontal Network for Affective Prosody. Front Hum Neurosci 4, 19, https://doi.org/10.3389/fnhum.2010.00019 (2010).
Wildgruber, D., Ethofer, T., Grandjean, D. & Kreifelts, B. A cerebral network model of speech prosody comprehension. International Journal of Speech-Language Pathology 11, 277–281 (2009).
Frühholz, S. et al. Asymmetrical effects of unilateral right or left amygdala damage on auditory cortical processing of vocal emotions. Proceedings of the National Academy of Sciences, 201411315 (2015).
Pannese, A., Grandjean, D. & Frühholz, S. Subcortical processing in auditory communication. Hearing research 328, 67–77 (2015).
Frühholz, S., Trost, W. & Kotz, S. A. The sound of emotions–Towards a unifying neural network perspective of affective sound processing. Neuroscience & Biobehavioral Reviews (2016).
Ratcliff, R. & Rouder, J. N. Modeling response times for two-choice decisions. Psychological Science 9, 347–356 (1998).
Summerfield, C. & Koechlin, E. A neural representation of prior information during perceptual inference. Neuron 59, 336–347 (2008).
Carrasco, M. Visual attention: The past 25 years. Vision research 51, 1484–1525 (2011).
Wyart, V., Nobre, A. C. & Summerfield, C. Dissociable prior influences of signal probability and relevance on visual contrast sensitivity. Proceedings of the National Academy of Sciences 109, 3593–3598 (2012).
McKenzie, C. R., Wixted, J. T., Noelle, D. C. & Gyurjyan, G. Relation between confidence in yes–no and forced-choice tasks. Journal of Experimental Psychology: General 130, 140 (2001).
Rao, R. P. Bayesian inference and attentional modulation in the visual cortex. Neuroreport 16, 1843–1848 (2005).
Bogacz, R., Brown, E., Moehlis, J., Holmes, P. & Cohen, J. D. The physics of optimal decision making: a formal analysis of models of performance in two-alternative forced-choice tasks. Psychological review 113, 700 (2006).
Brück, C., Kreifelts, B. & Wildgruber, D. Emotional voices in context: a neurobiological model of multimodal affective information processing. Physics of Life Reviews 8, 383–403 (2011).
Frühholz, S. et al. Asymmetrical effects of unilateral right or left amygdala damage on auditory cortical processing of vocal emotions. Proceedings of the National Academy of Sciences 112, 1583–1588 (2015).
Petrides, M. & Pandya, D. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. European Journal of Neuroscience 16, 291–310 (2002).
Petrides, M. Lateral prefrontal cortex: architectonic and functional organization. Philosophical Transactions of the Royal Society of London B: Biological Sciences 360, 781–795 (2005).
Rao, K. S., Koolagudi, S. G. & Vempada, R. R. Emotion recognition from speech using global and local prosodic features. International journal of speech technology 16, 143–160 (2013).
Koolagudi, S. G. & Krothapalli, R. S. Two stage emotion recognition based on speaking rate. International Journal of Speech Technology 14, 35–48 (2011).
Paulmann, S., Seifert, S. & Kotz, S. A. Orbito-frontal lesions cause impairment during late but not early emotional prosodic processing. Social Neuroscience 5, 59–75 (2010).
Tomasi, D., Ernst, T., Caparelli, E. C. & Chang, L. Common deactivation patterns during working memory and visual attention tasks: An intra‐subject fMRI study at 4 Tesla. Human brain mapping 27, 694–705 (2006).
Gusnard, D. A. & Raichle, M. E. Searching for a baseline: functional imaging and the resting human brain. Nature reviews. Neuroscience 2, 685 (2001).
Aron, A. R., Robbins, T. W. & Poldrack, R. A. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences 8, 170–177 (2004).
Levy, B. J. & Wagner, A. D. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Annals of the New York Academy of Sciences 1224, 40–62 (2011).
Kim, J.-N. & Shadlen, M. N. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nature neuroscience 2, 176–185 (1999).
Ratcliff, R. & McKoon, G. The diffusion decision model: theory and data for two-choice decision tasks. Neural computation 20, 873–922 (2008).
Cardoso‐Leite, P., Waszak, F. & Lepsien, J. Human perceptual decision making: Disentangling task onset and stimulus onset. Human brain mapping 35, 3170–3187 (2014).
Friston, K. A theory of cortical responses. Philosophical Transactions of the Royal Society of London B: Biological Sciences 360, 815–836 (2005).
de Lange, F. P., Jensen, O. & Dehaene, S. Accumulation of evidence during sequential decision making: the importance of top–down factors. Journal of Neuroscience 30, 731–738 (2010).
De Lange, F. P., Van Gaal, S., Lamme, V. A. & Dehaene, S. How awareness changes the relative weights of evidence during human decision-making. PLoS Biol 9, e1001203 (2011).
Kiani, R., Hanks, T. D. & Shadlen, M. N. Bounded integration in parietal cortex underlies decisions even when viewing duration is dictated by the environment. Journal of Neuroscience 28, 3017–3029 (2008).
Leitman, D. I. et al. “ It’s not what you say, but how you say it”: a reciprocal temporo-frontal network for affective prosody. Frontiers in human neuroscience 4, 19 (2010).
Kumar, S., von Kriegstein, K., Friston, K. & Griffiths, T. D. Features versus feelings: dissociable representations of the acoustic features and valence of aversive sounds. The Journal of Neuroscience 32, 14184–14192 (2012).
Frühholz, S. & Grandjean, D. Towards a fronto-temporal neural network for the decoding of angry vocal expressions. Neuroimage 62, 1658–1666 (2012).
Jabbi, M. et al. Convergent BOLD and beta-band activity in superior temporal sulcus and frontolimbic circuitry underpins human emotion cognition. Cerebral Cortex, bht427 (2014).
Frühholz, S. & Grandjean, D. Multiple subregions in superior temporal cortex are differentially sensitive to vocal expressions: a quantitative meta-analysis. Neuroscience & Biobehavioral Reviews 37, 24–35 (2013).
Juslin, P. N. & Laukka, P. Communication of emotions in vocal expression and music performance: Different channels, same code? Psychological bulletin 129, 770 (2003).
Sauter, D. A., Eisner, F., Calder, A. J. & Scott, S. K. Perceptual cues in nonverbal vocal expressions of emotion. The Quarterly Journal of Experimental Psychology 63, 2251–2272 (2010).
Greasley, P., Sherrard, C. & Waterman, M. Emotion in Language and Speech: Methodological Issues in Naturalistic Approaches. Language and Speech 43, 355–375 (2000).
Rosenberg, E. L. & Ekman, P. Conceptual and methodological issues in the judgment of facial expressions of emotion. Motivation and Emotion 19, 111–138 (1995).
Summerfield, C. & Koechlin, E. A neural representation of prior information during perceptual inference. Neuron 59, 336–347, https://doi.org/10.1016/j.neuron.2008.05.021 (2008).
Fruhholz, S., Klaas, H. S., Patel, S. & Grandjean, D. Talking in Fury: The Cortico-Subcortical Network Underlying Angry Vocalizations. Cerebral cortex (New York, N.Y.: 1991), https://doi.org/10.1093/cercor/bhu074 (2014).
Bänziger, T. & Scherer, K. R. Introducing the geneva multimodal emotion portrayal (gemep) corpus. Blueprint for affective computing: A sourcebook, 271–294 (2010).
Feinberg, D. A. et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PloS one 5, e15710 (2010).
Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113 (2007).
Glover, G. H., Li, T. Q. & Ress, D. Image‐based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine 44, 162–167 (2000).
Gläscher, J. & Gitelman, D. Contrast weights in flexible factorial design with multiple groups of subjects. SPM@ JISCMAIL. AC. UK) Sml, editor, 1–12 (2008).
Tzourio-Mazoyer, N. et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Friston, K. et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 6, 218–229 (1997).
Acknowledgements
The study was supported by a project grant of the Swiss National Science Foundation (SNSF 105314_146559/1) and the National Center for Affective Sciences (51NF40-104897). SF receives additional support from the SNSF (SNSF PP00P1_157409/1).
Author information
Authors and Affiliations
Contributions
M.D. and L.C. acquired and analyzed the data. M.D., D.G. and S.F. wrote the manuscript. S.F. designed the study.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dricu, M., Ceravolo, L., Grandjean, D. et al. Biased and unbiased perceptual decision-making on vocal emotions. Sci Rep 7, 16274 (2017). https://doi.org/10.1038/s41598-017-16594-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-16594-w
This article is cited by
-
Humans recognize affective cues in primate vocalizations: acoustic and phylogenetic perspectives
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.