Abstract
Vancomycin-resistant Enterococcus faecium is a leading cause of hospital-acquired infections and outbreaks. Regulatory RNAs (sRNAs) are major players in adaptive responses, including antibiotic resistance. They were extensively studied in gram-negative bacteria, but less information is available for gram-positive pathogens. No sRNAs are described in E. faecium. We sought to identify a set of sRNAs expressed in vancomycin-resistant E. faecium Aus0004 strain to assess their roles in daptomycin response and resistance. Genomic and transcriptomic analyses revealed a set of 61 sRNA candidates, including 10 that were further tested and validated by Northern and qPCR. RNA-seq was performed with and without subinhibitory concentrations (SICs) of daptomycin, an antibiotic used to treat enterococcal infections. After daptomycin SIC exposure, the expression of 260 coding and srna genes was altered, with 80 upregulated and 180 downregulated, including 51% involved in carbohydrate and transport metabolisms. Daptomycin SIC exposure significantly affected the expression of seven sRNAs, including one experimentally confirmed, sRNA_0160. We studied sRNA expression in isogenic mutants with increasing levels of daptomycin resistance and observed that expression of several sRNAs, including sRNA_0160, was modified in the stepwise mutants. This first genome-wide sRNA identification in E. faecium suggests that some sRNAs are linked to antibiotic stress response and resistance.
Similar content being viewed by others
Introduction
Enterococci are commensals of the gastrointestinal microbiota of many animal species1. Within the genus, Enterococcus faecalis and Enterococcus faecium have emerged as major opportunistic pathogens2. They have become resistant to numerous antibiotics3, with the spread of vancomycin-resistant enterococci (VRE), especially in E. faecium 4. The latter is part of the ESKAPE (E. faecium, S taphylococcus aureus, K lebsiella pneumoniae, A cinetobacter baumannii, P seudomonas aeruginosa and E nterobacter spp.) group of major multidrug-resistant (MDR) nosocomial pathogens5. E. faecium antimicrobial resistance is worrisome because of the dissemination of hospital-adapted clones belonging to the clonal complex 17 (CC17)6. Epidemic CC17 strains are part of a human hospital-adapted lineage (clade A1) that emerged from the animal-associated lineage (clade A2) after the introduction of antibiotics, and which differs genetically from the human community-associated lineage (clade B)7. Thanks to its huge genomic plasticity and metabolic versatility, E. faecium is a highly adapted commensal bacterial species that can turn into an opportunistic pathogen8. Despite their paramount importance, the mechanisms involved in this physiological transition have not been adequately investigated9.
Among the numerous environmental stresses with which bacteria must cope to survive, the presence of antibiotics, especially at subinhibitory concentrations (SICs, concentrations below the MIC, which do not significantly affect bacterial growth), is suspected to play a key role in the origin and evolution of antimicrobial resistance10. SICs are expected to occur during antibiotic treatment in humans, when drug diffusion at the infection site is inadequate or during exposure of the gastrointestinal tract microbiota to antibiotics11. Interestingly, a recent study has demonstrated that exposure to ciprofloxacin SICs enhance antimicrobial resistance and pathogenicity in E. faecium 12. Cyclic lipopeptide daptomycin is a commonly used antibiotic to treat vancomycin-resistant E. faecium (VREF) infections4, via a mechanism involving calcium-dependent interaction with the bacterial membrane that modifies its integrity and leads to cell death13. Daptomycin resistance in E. faecium is still rare, but treatment failures are increasingly reported, related to the emergence of high-level daptomycin resistance14, 15. Although several genes (e.g. liaFSR, yycFGHIJ, and cls) are known to be involved in the development of daptomycin resistance16, the different steps of resistance acquisition remain unclear, particularly those related to the selection of low-level resistant mutants17. Moreover, nothing is currently known about the stress response of E. faecium to daptomycin exposure.
In recent years, several studies have shown that antibiotic exposure is correlated with the expression of bacterial regulatory small RNAs (sRNA)18. These sRNAs are usually short (50–600 nts) noncoding transcripts synthesized under specific environmental conditions. Modulating the expression level of target genes, mainly post-transcription, to enable rapid, tight adaptation to cellular physiology, which includes pathogenicity and antimicrobial resistance19, they can either enhance resistance (e.g. MicF in E. coli 20) or increase susceptibility (e.g. SprX in S. aureus 21) to antibacterial agents. Recent in-depth transcriptomic analyses of several bacterial species, in the presence of antibiotic SICs, have revealed that antibiotic exposure significantly modifies the expression of numerous sRNAs22, 23.
Although many sRNAs have been identified in E. faecalis 24, 25, nothing is currently known about the E. faecium sRNome. This lack of knowledge is particularly detrimental because the worldwide ratio of E. faecalis/E. faecium infections is currently changing in favor of E. faecium 4. To search for sRNAs expressed in E. faecium, we performed a genome-wide transcriptome analysis (RNA-seq) onto a hospital-adapted CC17 VREF clinical isolate26. Using the DETR’PROK workflow, a pipeline devoted to sRNA identification in prokaryotes, combined with an in silico search for the conserved sRNAs in bacteria and RNA-seq depth testing within the intergenic regions (IGRs), we identified 61 sRNA candidates. Then we monitored the levels of all transcripts in bacterial cells grown with and without daptomycin SICs. Finally, we studied the transcript levels of these sRNAs in a series of isogenic mutants, with increasing levels of daptomycin resistance. Our study demonstrates that E. faecium expresses many sRNAs and that the expression of several of them is induced or repressed by antibiotic exposure or during development of resistance. Our finding that the expression of sRNA_0160 is downregulated by daptomycin exposure and repressed in daptomycin-resistant mutants implies that it is connected to antibiotic response and resistance in E. faecium. Accordingly, we plan to explore its function and molecular targets in the future.
Results
Identification of sRNAs in Enterococcus faecium
Thus far, no sRNA has been described in E. faecium. To investigate the presence of these molecules in this species, we studied the E. faecium Aus0004 reference strain, a vanB-positive CC17 clinical isolate recovered from a bloodstream infection in Australia27. This strain, containing a 2.9-Mb circular chromosome and three plasmids, is the first complete E. faecium genome to be sequenced27. This investigation used three different approaches: (i) the search for sRNAs already characterized in other bacteria by comparative genomics or from deep RNA-seq of E. faecium Aus0004, followed by the use of either (ii) the HTSeq/DESeq pipeline28, 29 or (iii) the DETR’PROK workflow30 (Fig. 1).
Identification of sRNA candidates in Enterococcus faecium. sRNAs were identified either by sequence homology with previously characterized sRNAs or from a deep RNA sequencing dataset. Intergenic regions (IGRs) of both DNA strands, with 50 nt removed at both ends to reduce false positives due to UTRs from adjacent genes, were extracted from the E. faecium Aus0004 genome with R scripts and gene annotation files. In a first curation step, IGRs with an HTSeq count <15 and an RPKM normalization <2 were discarded. In a second curation step, sRNA candidates (with adjusted coordinates obtained from both DETR’PROK and read mapping visualization) with an HTSeq count <15 and an RPKM normalization <3 were discarded. Finally, repeated sequences were identified by BlastN and removed during a final curation step. SRD, Staphylococcal regulatory RNA database (srd.genouest.org). BSRD, Bacterial small regulatory RNA Database (http://kwanlab.bio.cuhk.edu.hk/BSRD/); RPKM, Reads per kilobase per million mapped reads.
Seven sRNAs were identified by comparative sequence analysis with other gram-positive bacteria. Four of them shared similarities with cis-encoded riboswitches, while the others are related to the RNaseP RNA moiety or to tmRNA or 6 S RNA (Table 1). Using RNA-seq data collected from our transcriptomic analysis (see materials and methods), we obtained an initial set of 1,275 srna gene candidates, namely 1,035 from the HTSeq/DESeq pipeline and 240 from the DETR’PROK workflow (Fig. 1). From that set, we kept the candidates detected by both approaches and visualized their mapping patterns and adjacent environments using CLC Genomics, to discard untranslated regions (UTRs). This allowed us to reduce the list to 249 srna gene candidates. As shown in Fig. 1, we applied additional criteria to remove repeated sequences and candidates with weak expression profiles as described31. The nucleotide sequence of any candidate identified 10 times or more elsewhere in the Aus0004 genome was systematically discarded. To eliminate weakly expressed transcripts, some of which may have been due to background noise, we applied a stringent cut-off value (mean normalized count ≥10, with the DESeq package). This shortened the list to 54 sRNAs, which, together with the seven sequences retrieved by comparative genomics, produced a total of 61 candidates predicted to be sRNAs in E. faecium Aus0004. We used the DETR’PROK workflow to infer their nucleotide lengths from the RNA-seq data (Fig. 1 and Table 1).
E. faecium sRNA conservation and experimental validation
We then analyzed the 61 sRNAs initially identified. Figure 2 depicts the genomic location of these transcripts. Note that three srna genes were part of prophages or genomic islands, according to the annotation performed by Lam et al.27: two (sRNA_1930 and sRNA_1940) in prophage phiEnfa003, and one (sRNA_1300) in an annotated 60-kb genomic island (Fig. 2).
RNA-seq E. faecium genome annotation discovers 61 new expressed transcripts. The mean normalized count, calculated with the DESeq package, of gene expression is indicated as a black (prophages and Genomic Islands) or red (rest of the genome) line in the gray circle, and thin gray circular lines represent the mean expression level of each gene as a Log2 value (Table S3). The outermost circle represents the full E. faecium Aus0004 genome with a 30-fold magnification of the 61 candidate sRNAs. Candidate sRNAs_1300, _1930 and _1940 (in bold) are located in prophages and Genomic Islands according to the annotation by Lam et al.27.
The conservation of these 61 sRNAs candidates among 86 fully E. faecium sequenced genomes was examined (Fig. 3). Importantly, three clades were identified within the species E. faecium, (clades A1, A2 and B) as inferred by comparative genomics7, 32. Out of the 61 srna candidates, 32 (52%) were conserved among all E. faecium strains while 13 were absent in clade B strains (Supplementary Table S1). No gene was uniquely found either in clades A1 or A2 while none was specific to Aus0004 (Supplementary Table S1). Of interest, the median number of srna gene candidates was significantly higher in clade A1 strains than in clades A2 and B (P < 0.0001) (Supplementary Fig. S1).
Comparative analysis of the presence and absence of 61 novel sRNAs candidates in E faecium strains. A heat map was generated based on the presence (red) and absence (white) of 61 expressed srna genes in the 86 fully sequenced E. faecium strains that belong to the different phylogenetic clades. Clades A1, A2 and B (according to7) are indicated in magenta, blue and green, respectively.
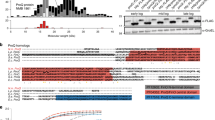
Analysis of sRNA expression further strengthened our transcriptomic analysis. Ten of the 61 sRNA candidates, selected mainly based on their high levels of expression, inferred from the RNA-seq data (Table 1), were challenged experimentally by Northern blots, including tmRNA (ssrA) (subsequently used as an internal control) (Fig. 4). This technique enabled us to monitor the expression of these 10 sRNAs at three time points (ME, mid-exponential; LE, late-exponential; ES, early-stationary) during bacterial growth (Fig. 4). Almost all were expressed at the ME and LE phases, while three (sRNA_1300, sRNA_2050 and sRNA_2210) were not detected at the ES phase (Fig. 4). The nucleotide length of each sRNA was also estimated, based on their PAGE migration, combined with the use of a pre-stain RNA ladder (75 and 100 nt) mixed with tmRNA (366 nt). An excellent match was observed between the data obtained by Northern blots and the predicted sizes based on the RNA-seq data. Three expressed sRNAs (sRNA_0030, sRNA_2210, and sRNA_2410) were produced as two transcripts (Fig. 4).
Experimental validation of 10 sRNAs expressed from E. faecium Aus0004 and the impact of daptomycin SIC on their expression. Northern blots were performed on RNAs extracted from cells collected at an OD600 nm of 0.7, 1.6, and 1.9 corresponding to mid (ME), late (LE) exponential and early stationary (ES) phases of growth. The RNAs were extracted on cells grown in the absence (D−) or presence (D+) of daptomycin SIC. tmRNA levels were used as internal loading controls. The data show a representative experiment among three independent biological replicates.
Genome-wide transcriptomic analysis after exposure to daptomycin subinhibitory concentrations
Our search for sRNAs expressed by E. faecium included a global transcriptomic analysis. Using RNA-seq, we compared the transcriptome, including both mRNAs and sRNAs of E. faecium Aus0004 cultured with ( + Dap) or without (-Dap) a SIC (0.5 μg/ml corresponding to 1/4 MIC) of daptomycin, an antibiotic commonly used to treat VREF infections.
Between 23 and 33 million reads were obtained for each stranded cDNA library made from total RNAs collected at the LE growth phase; 54–75% mapped to the genome of E. faecium Aus0004, corresponding to average genome coverage of 255 to 417 (Supplementary Table S2). Note that rRNA depletion was highly efficient, with only 1 to 9% of reads corresponding to rRNA genes (Supplementary Table S2). The reproducibility of experimental duplicates was very satisfactory in both conditions (r2 = > 0.97, Supplementary Fig. S2). Between 38 and 72% of reads mapped to coding sequences (CDSs), and 1 to 6 million reads to sRNA candidates (Supplementary Table S2). Experimental challenge by RT-qPCR of gene expression variations detected by RNA-seq, with a Pearson correlation coefficient of 0.9592 (Fig. 5).
Genome-wide transcriptional response of E. faecium Aus0004 to daptomycin SIC. (a) Global analysis of transcript levels in E. faecium Aus0004 by RNA-seq. Conditions and ‘+Dap’ and ‘−Dap’ refer to bacterial growth in the presence or absence of daptomycin (concentration at 0.5 μg/mL), respectively. Blue and green inner circles correspond to the mean expression of each gene, as calculated by DESeq, in bacteria grown under ‘−Dap’ and ‘+Dap’ conditions, respectively. The red line in the gray circle represents the baseline, and thin gray circular lines represent four-fold (or log2 = 2) changes in expression of each gene (Supplementary Table S3). The outermost circle represents the full E. faecium Aus0004 genome with a 20-fold magnification of the genes for which expression was confirmed by qRT-PCR. (b) Validation of the RNA-seq data by qRT-PCR for 10 selected genes. Mean log2 ratios of values determined in the qRT-PCR experiments are plotted against the mean log2 ratios of the RNA-seq experiments.
Overall transcriptome picture
Figure 5 and Supplementary Table S3A present the transcriptome expression levels of cells grown under −Dap and +Dap conditions. Under daptomycin SIC exposure, 280 genes presented significantly altered transcript levels (fold change of expression <−4 or >4, adjusted P-value < 0.05), including 80 upregulated and 180 downregulated genes (Supplementary Table S3B and C). Among these 280 genes, 7 (3 downregulated and 4 upregulated) corresponded to sRNA candidate genes that we identified earlier (Supplementary Tables S3B and C). To interpret the RNA-seq data, we performed a COG (Clusters of Orthologous Groups of proteins) functional categorization to evaluate the impacted metabolic pathways (Supplementary Fig. S3)33. In the presence of a subinhibitory daptomycin concentration, we observed significant induction of genes coding for proteins involved in nucleotide metabolism and transport, transcription, replication and repair, and cell wall/membrane/envelope biogenesis(Supplementary Fig. S3). Genes coding for proteins involved in energy production and conversion and in carbon metabolism and transport were significantly repressed (Supplementary Fig. S3). Carbon metabolism and transport activity decreased strongly, with 51% (91/180) of the repressed genes belonging to this functional category (Supplementary Table S3C).
Differentially expressed mRNAs and sRNAs
The most significant variation of gene expression caused by a subinhibitory daptomycin concentration concerned genes involved in galactose metabolism (EFAU004_00481-EFAU004_00483), with a decrease ranging from −87 to −112 fold (Supplementary Table S3C). This finding suggests that the stress caused by the presence of a low antibiotic concentration deeply modifies the carbon flow, with concomitant alteration of interconnected metabolic pathways and specific use of energy sources available for growth.
Supplementary Table S4 reports the modulation in expression of the genes characterized as related to virulence and antimicrobial resistance4, 34 on daptomycin exposure, based on this transcriptomic study. Among the 24 potential virulence genes described in E. faecium Aus0004, four showed significant change in expression (fold change <−4 or >4, adjusted P-value < 0.05), with three genes repressed and only one induced. The acm gene, coding for the main collagen-binding adhesin in E. faecium, was significantly upregulated by a magnitude of 7.8-fold in the presence of daptomycin SIC (Supplementary Table S4). This induction was phenotypically confirmed by collagen-binding assays (Supplementary Fig. S4A), since Acm binds collagen type I and type IV35. By contrast, the level of biofilm production did not differ significantly with or without daptomycin (Supplementary Fig. S4B), a finding consistent with the lack of significant change in the expression of the major factors involved in biofilm formation (i.e. ebpABC, empABC and esp 36,37,38) (Supplementary Table S4). Among the repressed genes were two encoded carbohydrate phosphotransferase system (PTS) proteins, ptsD and bepA, which had fold changes of −7.5 and −10.1, respectively (Supplementary Table S4). Interestingly, both PTS proteins are involved in E. faecium pathogenesis, that is, absent in human commensal isolates and enriched in isolates responsible for hospital outbreaks and infections39, 40. Significant downregulation was also observed in levels of swpA, which expresses a protein containing a WxL domain, which in turn plays a role in bile salt stress and endocarditis pathogenesis41. No resistance genes showed any significant expression change (fold change <−4 or >4, adjusted P-value < 0.05, Supplementary Table S4).
Bacterial sRNAs participate in the regulation of physiological networks and adaptation to specific modifications of environmental conditions, including antibiotic exposure22. In addition to the gene-coding variations detected, daptomycin exposure significantly modulated the expression of 7 srna genes, among the 61 candidates (fold change of expression <−4 or >4, adjusted P-value < 0.05), upregulating 3 sRNAs (sRNA_0560, +4.9; sRNA_1420, +11.2; sRNA_2290, +6.3) and downregulating 4 (sRNA_0160, −11.2; sRNA_0170, −6.2; sRNA_0290, −5.4; 6 S RNA, −5.9).
The expression of the experimentally validated sRNAs under daptomycin SIC was assessed at three time points during growth by Northern blots (Fig. 4) and qPCRs (Supplementary Fig. S5). These data were compared to those obtained by RNA-seq with RNAs extracted at the LE growth phase and showed satisfactory agreement (Table 2). The transcript level of only one sRNA (sRNA_0160) decreased significantly under antibiotic exposure (fold change of −11.2), and none increased (Table 2). This experimental evidence provides us substantial confidence in the specific responses of this sRNA to daptomycin stress. At the three time points measured during E. faecium growth under daptomycin exposure, the expression of four sRNAs remained mostly uniform (sRNA_1260, sRNA_1300, sRNA_2050 and sRNA_2410), whereas that of five sRNAs (sRNA_0030, sRNA_0120, sRNA_0160, sRNA_0280 and sRNA_2210) fluctuated substantially (Supplementary Fig. S5).
Potential roles of experimentally validated sRNAs in daptomycin resistance
Further experiments used a series of incremental daptomycin-resistant mutants (named Mut4 to Mut128) to investigate the link between the expression of the nine experimentally validated sRNAs (all except tmRNA) and E. faecium daptomycin resistance. These mutants were previously obtained in vitro from E. faecium strain Aus0004 and have daptomycin MICs ranging from between 4 to 128 mg/L, while the MIC of the parental strain was 2 mg/L17. After extracting total RNAs at the LE phase, we studied the expression of these nine sRNAs with qPCR. Expression of three sRNAs (sRNA_0160, sRNA_1260, sRNA_2050) was modified sharply in the stepwise daptomycin-resistant mutants (Fig. 6), with sRNA_0160 repressed in Mut8 to Mut128 (fold changes from −36 to −4), sRNA_1260 (fold changes from + 8 to + 36), and sRNA_2050 (fold changes from + 3 to + 13) induced over the series (Fig. 6). This finding suggests that these sRNAs play a role in the progressive acquisition of daptomycin resistance by E. faecium.
sRNA expression in isogenic stepwise daptomycin-resistant mutants of E. faecium Aus0004. A series of in vitro mutants derived from strain E. faecium Aus0004 with increasing daptomycin MICs (4, 8, 18, 32, 64, and 128 μg/mL vs. 2 μg/mL for the parental strain)17 was tested. qRT-PCR experiments were performed in triplicates on RNAs extracted from cells collected at an OD600 nm of 1.6 (late exponential growth phase).
Discussion
Many studies have identified sRNAs in a multitude of bacterial genomes, but mainly in gram-negative bacteria. In recent years, however, large numbers of sRNAs have been identified in a dozen gram-positive bacteria42, including E. faecalis 24,25,26, 43. Enterococci are highly adapted members of the intestinal microbiota of a range of hosts spanning the animal kingdom8. They are also leading opportunistic hospital pathogens that cause infections often resistant to many therapeutic options.
Two species, E. faecalis and E. faecium, cause the vast majority of hospital-acquired enterococcal infections in humans44. Unfortunately, the knowledge acquired about the sRNome of E. faecalis cannot be translated to E. faecium since these two species are at opposite ends of the phylogenetic tree8: E. faecalis occurs in one of the oldest branches of the genus, whereas E. faecium arose more recently. Of these two, it is E. faecium that has most often acquired resistance to several classes of antibiotics, including vancomycin45. In fact, epidemic hospital-adapted CC17 strains, such as E. faecium Aus0004, are part of a human hospital-adapted lineage (clade A1) that emerged approximately 75 years ago from the animal-associated lineage (clade A2) after the introduction of antibiotics: both differ genetically from the human community-associated lineage (clade B)7. These successful hospital-adapted strains have the ability to acquire adaptive elements cumulatively by horizontal gene transfer, a feature known as “genetic capitalism” and associated with the emergence of high-risk MDR clones46. The larger genome observed in clade A1 strains compared with those in clades A2 and B7 suggests that they harbor more coding genes. Even if the experimental validation of all 61 candidate genes would be necessary, our data suggest that it is also likely true for the non-coding sRNA genes. Indeed, strains from clade B harbor much less srna genes (median number = 39) than strains belonging to clades A1 (median number = 54) and clade A2 (median number = 50).
Bioinformatic tools combined with experimental analyses enabled us to identify 61 sRNA candidates from the genome of E. faecium Aus0004, a clade A1 strain. These srna genes are scattered throughout the entire bacterial chromosome, including in pathogenicity islands. We are aware that this pioneering study was performed only in one strain, a vanB VRE clinical isolate. We should extend our investigations in other E. faecium genetic backgrounds that may reveal discrepancies in their overall sRNA contents. Two thirds of them were detected in all of the E. faecium strains that have so far been fully sequenced. Most of these srna genes (excluding tmRNA, RNase P RNA and sRNA_0030), however, have no sequence homologs in E. faecalis. This finding may imply that enterococci possess their own set of sRNAs, as observed for S. aureus and other staphylococci47. Their predicted lengths, ranging from 67 to 437 nts (Table 1), are compatible with their being sRNAs48. As suspected, these bacterial species express tmRNA, which is required for ribosome rescue during translation of faulty mRNAs49, RNase P RNA for 5′-end maturation of tRNAs50, and 6S RNA, which interacts with the primary holoenzyme form of RNA polymerase to influence transcription51. Strikingly, 6S RNA expression decreases 6-fold on daptomycin exposure; the biological link thus revealed between 6S RNA and antibiotic response in this bacterial strain requires further investigation. Also of interest is sRNA_0030, whose nucleotide sequence was identified, with an identical nucleotide sequence, in different firmicutes (Streptococcus, Lactobacillus, Staphylococcus, Clostridium and Listeria), as well as plasmids from gram-negative bacteria (Neisseria gonorrhoeae and Escherichia coli) and mycoplasmas (Ureaplasma urealyticum). sRNA_0030 is expressed from an integrative transposon of the Tn916 family carrying the tet(M) gene, which confers tetracycline resistance52, 53. It is expressed 30 to 50 nts upstream from the initiation codon of tet(M) in various strains from the firmicutes bacterial phylum, including S. aureus, suggesting that it may be involved into regulation of tetracycline resistance, with no implications into daptomycin exposure and resistance.
An in-depth expression study was carried out on the most expressed sRNAs that were confirmed by three independent experimental evidences including RNA-seq, qPCR and Northern blots. All sRNAs possess specific expression profiles during E. faecium growth, some accumulating early on (sRNA_1260), or at later growth stages (sRNA_0160), reminiscent with their regulatory functions.
Daptomycin is a lipopeptide antibiotic with bactericidal activity against gram-positive bacteria, including multidrug-resistant nosocomial pathogens such as methicillin-resistant S. aureus (MRSA) and VRE54. It inserts into the bacterial cell membrane by a calcium-dependent mechanism13, 55 and forms oligomeric pores, which results in ion leakage and membrane potential dissipation56. In E. faecium, daptomycin resistance results from alteration of regulatory systems involved in the bacterial cell envelope stress response, as liaFSR, and enzymes involved in phospholipid metabolism, as cls 16.
Transcriptomic analysis was performed using reference strain E. faecium Aus0004, a vanB-positive clinical isolate belonging to clade A127. This strain has a 2.9-Mb circular chromosome composed of 2,753 ORFs, including several virulence factors such as enterococcal surface protein (esp) and collagen-binding adhesin (acm). This study has revealed up and down regulations of a small subset of virulence genes in E. faecium under daptomycin exposure. Indeed, acm was significantly upregulated, with a magnitude fold of 7.8 (Supplementary Table S4). Daptomycin-induced expression was phenotypically confirmed by collagen-binding assays (Supplementary Fig. S4A). Recently, we demonstrated such antibiotic-dependent upregulation of acm in the presence of ciprofloxacin SIC12. Since Acm is a primary collagen adhesin involved in experimental infective endocarditis57, acm induction caused by antibiotics SICs may be clinically relevant. By contrast, ptsD (−7.5-fold change) and bepA (−10.1-fold change) virulence genes that contribute to intestinal colonization and endocarditis/biofilm formation, respectively,39, 40 were significantly downregulated. Even though bepA is implicated in biofilm formation, there was no significant change in biofilm construction in the presence of daptomycin SIC (Supplementary Fig. S4B). However, numerous additional genes are involved in biofilm production and regulation in E. faecium, such as ebpABC/empABC, esp, asrR, ebrB, atlA Efm , sgrA, capD 35,36,37,38, 58,59,60, implying that biofilm regulation in this bacterium is a complex and multi-component process, as for many other bacteria61. Because several sRNAs are involved in the regulation of biofilm formation in a variety of bacteria62, a reasonable hypothesis is that some of the riboregulators described here could also be involved. Using stringent cut-off values for transcriptomic analysis (i.e. fold change <−4 or > 4 and P < 0.05), no resistance genes showed significant change in expression level. However, genes involved in daptomycin resistance, such as liaFSR and cls 16 had some degree of changes in expression (Supplementary Table S4). It is noteworthy since expression level of the liaFSR operon is linked to daptomycin resistance levels17.
A growing number of sRNAs are implicated in bacterial antibiotic resistance18, although physiological and molecular explanations of their involvement is largely unknown. In other gram-positive human pathogens such as S. aureus, some sRNAs are part of a coordinated transcriptional response to specific antimicrobial exposures22, or are involved in glycopeptide resistance21. The expression level of one sRNA (sRNA_0160) was significantly downregulated under daptomycin exposure. In addition, sRNA_0160 was also significantly repressed in daptomycin-resistant mutants. The pathways leading to daptomycin-resistance selection in vitro, however, may not entirely represent the process that occurs in vivo under daptomycin therapy, thus these data may not be conclusive in clinics. Taken together, it suggests that sRNA_0160 would be connected to antibiotic response and resistance in E. faecium, and therefore further investigations regarding the functions, mechanisms and molecular targets of this riboregulator will be conducted.
Concluding remarks
Our study demonstrates the existence of sRNAs expressed by E. faecium, a notorious ESKAPE opportunistic human pathogen. These novel sRNAs could be included, in the future, in a new resource for the hundreds of sRNAs identified in gram-positive bacteria63, as recently documented for staphylococci47. The 10 most expressed sRNAs expressed by E. faecium were investigated further. The expression of some sRNAs is induced upon daptomycin SIC exposure, and their possible connections with antibiotic resistance acquisition were identified. The set of daptomycin-responsive genes, including several virulence genes and riboregulators, was identified and some phenotypes validated experimentally. It is anticipated that such a detailed inventory of transcription units and sRNAs will provide substantial assistance for future investigations of this major cause of hospital-acquired human infections worldwide.
Methods
Bacterial strains, growth conditions, and antimicrobial susceptibility testing
E. faecium strain Aus000427 and isogenic daptomycin-resistant mutants17 were grown at 37°C in Brain Heart Infusion broth (BHI) or on agar plates (Becton Dickinson). Minimal inhibitory concentrations (MICs) of daptomycin were determined in triplicates on Mueller-Hinton agar with E-test strips (bioMérieux, Marcy l'Etoile, France). The growth kinetics of E. faecium strain Aus0004 were assessed in vitro in BHI broth with increasing (0.06 to 2 µg/ml) daptomycin concentrations at 37°C for a 24-hour period, and the experiments were performed in triplicates. The subinhibitory concentration (SIC) corresponded to the highest antibiotic concentration with no significant effects for bacterial growth.
RNA-seq analysis and RT-qPCR validation
E. faecium strain Aus0004 was cultured at 37°C until the late exponential growth phase (OD 1.6) in BHI broth (adjusted to 50 µg/ml Ca2+), with and without daptomycin at SIC, and total RNA was extracted with the ZR Fungal/Bacterial RNA Miniprep kit (Zymo Research, Irvine, CA). Residual chromosomal DNA was removed by treating samples with the TURBO DNA-free kit (Life Technologies, Saint Aubin, France). Samples were quantified with a Biospec-Nano spectrophotometer (Shimadzu, Noisiel, France), and their integrity was assessed with the Agilent 2100 bioanalyzer. A Ribo-ZeroTM Magnetic kit from gram-Positive Bacteria (Epicentre, France) was used to remove the 23S, 16S and 5S rRNAs from the samples. rRNA depletion was verified on an Agilent 2100 bioanalyzer. The next steps, from mRNA fragmentation to high-throughput sequencing, were performed by ProfileXpert (Lyon, France). The library was constructed with the dUTP-Based NEXTflex™ Directional RNA-Seq Kit, and the samples were sequenced on an Illumina Hi-Seq 2500 platform (single-end, 50 cycles). The experiments were done in duplicates. The COG analysis was performed using updated database64. For the reverse transcription-qPCR experiments challenging the differentially expressed mRNAs, cDNA was synthesized from 25 ng total RNA with a QuantiTect RT kit (Qiagen, Courtaboeuf, France). Transcript levels were confirmed by the ΔΔCt method with adk as the housekeeping control gene12 (Supplementary Table S5). These experiments were performed in triplicates.
Candidate sRNA identification and conservation analysis
The E. faecium Aus0004 genome sequence and annotation files were obtained from NCBI at: ftp://ftp.ncbi.nlm.nih.gov/genomes/Bacteria/. Two approaches were used to identify candidate sRNAs in the E. faecium genome. First, the updated annotation files (in GFF format) including all of the intergenic regions and the antisense portions of the coding genes, were created. The Fastq files were mapped onto the bacterial genomic sequence with BWA65. The reads were counted by HTSeq count28 with the GFF files. An RPKM normalization procedure was applied, and all the sRNA candidates with an RPKM <3 and HTseq-count <15 were removed. The retained candidates were submitted to Rfam database66 and bacterial sRNA databases (BSRD, SRD), which allowed us to identify and keep the initial nomenclature of seven srna genes (sefa2424.1T-box, sefa2004.1T-box, sefa1356.1T-box, RNase P, sefa1496.1FMN, tmRNA, 6S RNA), and we then confirmed this prediction with Infernal67. In the second approach, DETR’PROK30, a workflow devoted to prokaryotic sRNA identification, was applied to our dataset with the standard annotation downloaded from NCBI. From BWA alignments, DETR’PROK clustered reads located within non-annotated regions of the E. faecium Aus0004 genome. The workflow was set to retain all clusters containing more than 50 nucleotides, more than 12 reads, and located at least 25 nucleotides apart from any coding sequence as described31. The outputs obtained from both methods were compiled, to produce a list of the sRNA candidates detected by both approaches. The criteria of sRNA conservation analysis among the E. faecium strains was performed using BlastN with 70% identity and 60% sequence coverage.
Candidate sRNA experimental assessment by Northern blot and qPCR
RNA extractions were performed at three time points during growth – middle exponential (ME, OD 0.7), late exponential (LE, OD 1.6), and early stationary (ES, OD 1.9). Extractions were performed as reported31. Cell pellets were dissolved into 500 µL of lysis buffer, and cells were broken by acid-treated glass beads and phenol. Total RNA was extracted by phenol/chloroform and ethanol precipitated overnight. RNA samples (15 μg) were loaded on denaturing 7.5% PAGE and transferred onto Zeta probe GT membranes (Bio-Rad) in 0.5 × TBE. Membranes were hybridized with specific 32P-labeled probes in ExpressHyb solution (Clontech, USA), washed, exposed, and scanned onto a PhosphorImager (Molecular Dynamics). For the RT-qPCR experiments, cDNA synthesis was performed with the High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) and quantitative PCR with the Power Sybr® Green PCR Master Mix (Thermofisher Scientific). Transcript levels were determined by the ΔΔCt method, with adk as the control (Supplementary Table S5). Each experiment was performed in triplicates.
Collagen binding and biofilm formation assays
High-binding microtiter plate wells (Immulon 2 HB, Corning) were coated overnight at 4 °C with collagen at 10 μg/mL (from human fibroblast, Sigma-Aldrich, France) or bovine serum albumin (BSA, Sigma-Aldrich, France) as negative controls. After washing the wells, the remaining protein-binding sites were blocked by 1% PBS-BSA for one hour. Two E. faecium Aus0004 cultures were grown overnight, one with and one without daptomycin SIC, in BHI adjusted to 50 µg/ml Ca2+. Cells were centrifuged and re-suspended into 1 mL PBS; 100 µL of cells (108 CFU/mL) was added to the wells and incubated at 37 °C for 2, 6, and 18 hours. The wells were carefully washed four times at each time point with 100 µL PBS. To recover the E. faecium cells bound to collagen, the wells were scratched and re-suspended into 100 µL PBS. Serial cell dilutions and inoculations onto BHI agar enabled us to count the adherent bacteria on collagen.
The biofilm formation was measured as previously described9. Each assay was repeated three times in at least three independent experiments. Statistical comparison conditions at each time point was performed using the unpaired t test.
References
Macovei, L. & Zurek, L. Ecology of antibiotic resistance genes: characterization of enterococci from houseflies collected in food settings. Appl Environ Microbiol 72, 4028–4035 (2006).
van Harten, R. M., Willems, R. J., Martin, N. I. & Hendrickx, A. P. Multidrug-Resistant Enterococcal Infections: New Compounds, Novel Antimicrobial Therapies? Trends Microbiol. doi:10.1016/j.tim.2017.01.004 (2017).
Arias, C. A. & Murray, B. E. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10, 266–278 (2012).
Cattoir, V. & Giard, J. C. Antibiotic resistance in Enterococcus faecium clinical isolates. Expert Rev Anti Infect Ther 12, 239–248 (2014).
Boucher, H. W. et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48, 1–12, doi:10.1086/595011 (2009).
Willems, R. J. et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 11, 821–828 (2005).
Lebreton, F. et al. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. MBio 4, doi:10.1128/mBio.00534-13 (2013).
Van Tyne, D. & Gilmore, M. S. Friend turned foe: evolution of enterococcal virulence and antibiotic resistance. Annu Rev Microbiol 68, 337–356, doi:10.1146/annurev-micro-091213-113003 (2014).
Lebreton, F. et al. AsrR is an oxidative stress sensing regulator modulating Enterococcus faecium opportunistic traits, antimicrobial resistance, and pathogenicity. PLoS Pathog 8, e1002834 (2012).
Davies, J. & Davies, D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74, 417–433, doi:10.1128/MMBR.00016-10 (2010).
Andersson, D. I. & Hughes, D. Microbiological effects of sublethal levels of antibiotics. Nat Rev Microbiol 12, 465–478 (2014).
Sinel, C. et al. Subinhibitory concentrations of ciprofloxacin enhance antimicrobial resistance and pathogenicity of Enterococcus faecium. Antimicrob Agents Chemother, doi:10.1128/AAC.02763-16 (2017).
Jung, D., Rozek, A., Okon, M. & Hancock, R. E. Structural transitions as determinants of the action of the calcium-dependent antibiotic daptomycin. Chem Biol 11, 949–957, doi:10.1016/j.chembiol.2004.04.020 (2004).
Lewis, J. S. 2nd et al. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother 49, 1664–1665 (2005).
Kelesidis, T., Tewhey, R. & Humphries, R. M. Evolution of high-level daptomycin resistance in Enterococcus faecium during daptomycin therapy is associated with limited mutations in the bacterial genome. J Antimicrob Chemother 68, 1926–1928 (2013).
Tran, T. T., Munita, J. M. & Arias, C. A. Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci 1354, 32–53, doi:10.1111/nyas.12948 (2015).
Sinel, C. et al. Sequential steps of daptomycin resistance in Enterococcus faecium and reversion to hypersusceptibility through IS-mediated inactivation of the liaFSR operon. J Antimicrob Chemother 71, 2793–2797, doi:10.1093/jac/dkw229 (2016).
Lalaouna, D., Eyraud, A., Chabelskaya, S., Felden, B. & Masse, E. Regulatory RNAs involved in bacterial antibiotic resistance. PLoS Pathog 10, e1004299, doi:10.1371/journal.ppat.1004299 (2014).
Wagner, E. G. & Romby, P. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv Genet 90, 133–208 (2015).
Kim, T., Bak, G., Lee, J. & Kim, K. S. Systematic analysis of the role of bacterial Hfq-interacting sRNAs in the response to antibiotics. J Antimicrob Chemother 70, 1659–1668, doi:10.1093/jac/dkv042 (2015).
Eyraud, A., Tattevin, P., Chabelskaya, S. & Felden, B. A small RNA controls a protein regulator involved in antibiotic resistance in Staphylococcus aureus. Nucleic Acids Res 42, 4892–4905 (2014).
Howden, B. P. et al. Analysis of the small RNA transcriptional response in multidrug-resistant Staphylococcus aureus after antimicrobial exposure. Antimicrob Agents Chemother 57, 3864–3874 (2013).
Stubben, C. J. et al. Differential expression of small RNAs from Burkholderia thailandensis in response to varying environmental and stress conditions. BMC Genomics 15, 385 (2014).
Shioya, K. et al. Genome-wide identification of small RNAs in the opportunistic pathogen Enterococcus faecalis V583. PLoS One 6, e23948 (2011).
Michaux, C. et al. Involvement of Enterococcus faecalis small RNAs in stress response and virulence. Infect Immun 82, 3599–3611, doi:10.1128/IAI.01900-14 (2014).
Fouquier d’Herouel, A. et al. A simple and efficient method to search for selected primary transcripts: non-coding and antisense RNAs in the human pathogen Enterococcus faecalis. Nucleic Acids Res 39, e46, doi:10.1093/nar/gkr012 (2011).
Lam, M. M. et al. Comparative analysis of the first complete Enterococcus faecium genome. J Bacteriol 194, 2334–2341 (2012).
Anders, S., Pyl, P. T. & Huber, W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169, doi:10.1093/bioinformatics/btu638 (2015).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol 11, R106, doi:10.1186/gb-2010-11-10-r106 (2010).
Toffano-Nioche, C. et al. Detection of non-coding RNA in bacteria and archaea using the DETR’PROK Galaxy pipeline. Methods 63, 60–65 (2013).
Bronsard, J. et al. sRNA and cis-antisense sRNA identification in Staphylococcus aureus highlights an unusual sRNA gene cluster with one encoding a secreted peptide. Sci Rep. 7, 4565 (2017).
Palmer, K. L. et al. Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. MBio 3, e00318–00311, doi:10.1128/mBio.00318-11 (2012).
Tatusov, R. L. et al. The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acids Res 29, 22–28 (2001).
Goh, H. M. S., Yong, M. H. A., Chong, K. K. L. & Kline, K. A. Model systems for the study of Enterococcal colonization and infection. Virulence 19, 1–38 (2017).
Paganelli, F. L. et al. Enterococcus faecium biofilm formation: identification of major autolysin AtlAEfm, associated Acm surface localization, and AtlAEfm-independent extracellular DNA Release. MBio 4, e00154, doi:10.1128/mBio.00154-13 (2013).
Leavis, H. et al. A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J Bacteriol 186, 672–682 (2004).
Sillanpaa, J. et al. Characterization of the ebp(fm) pilus-encoding operon of Enterococcus faecium and its role in biofilm formation and virulence in a murine model of urinary tract infection. Virulence 1, 236–246 (2010).
Montealegre, M. C. et al. Role of the Emp Pilus Subunits of Enterococcus faecium in Biofilm Formation, Adherence to Host Extracellular Matrix Components, and Experimental Infection. Infect Immun 84, 1491–1500, doi:10.1128/IAI.01396-15 (2016).
Zhang, X. et al. Identification of a genetic determinant in clinical Enterococcus faecium strains that contributes to intestinal colonization during antibiotic treatment. J Infect Dis 207, 1780–1786, doi:10.1093/infdis/jit076 (2013).
Paganelli, F. L. et al. Genome-wide Screening Identifies Phosphotransferase System Permease BepA to Be Involved in Enterococcus faecium Endocarditis and Biofilm Formation. J Infect Dis 214, 189–195, doi:10.1093/infdis/jiw108 (2016).
Galloway-Pena, J. R. et al. The identification and functional characterization of WxL proteins from Enterococcus faecium reveal surface proteins involved in extracellular matrix interactions. J Bacteriol 197, 882–892, doi:10.1128/JB.02288-14 (2015).
Brantl, S. & Bruckner, R. Small regulatory RNAs from low-GC Gram-positive bacteria. RNA Biol 11, 443–456, doi:10.4161/rna.28036 (2014).
Livny, J., Teonadi, H., Livny, M. & Waldor, M. K. High-throughput, kingdom-wide prediction and annotation of bacterial non-coding RNAs. PloS one 3, e3197, doi:10.1371/journal.pone.0003197 (2008).
Agudelo Higuita, N. I. & Huycke, M. M. in Eerococci: From Commensals to Leading Causes of Drug Resistant Infection (eds M. S. Gilmore, D. B. Clewell, Y. Ike, & N. Shankar) (2014).
O’Driscoll, T. & Crank, C. W. Vancomycin-resistant enterococcal infections: epidemiology, clinical manifestations, and optimal management. Infect Drug Resist 8, 217–230, doi:10.2147/IDR.S54125 (2015).
Guzman Prieto, A. M. et al. Global Emergence and Dissemination of Enterococci as Nosocomial Pathogens: Attack of the Clones? Front Microbiol 7, 788, doi:10.3389/fmicb.2016.00788 (2016).
Sassi, M. et al. SRD: a Staphylococcus regulatory RNA database. Rna 21, 1005–1017, doi:10.1261/rna.049346.114 (2015).
Jagodnik, J., Brosse, A., Le Lam, T. N., Chiaruttini, C. & Guillier, M. Mechanistic study of base-pairing small regulatory RNAs in bacteria. Methods. doi:10.1016/j.ymeth.2016.09.012 (2016).
Buskirk, A. R. & Green, R. Ribosome pausing, arrest and rescue in bacteria and eukaryotes. Philos Trans R Soc Lond B Biol Sci 372, doi:10.1098/rstb.2016.0183 (2017).
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35, 849–857 (1983).
Cavanagh, A. T. & Wassarman, K. M. 6S RNA, a global regulator of transcription in Escherichia coli, Bacillus subtilis, and beyond. Annu Rev Microbiol 68, 45–60, doi:10.1146/annurev-micro-092611-150135 (2014).
Roberts, A. P. & Mullany, P. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol Rev 35, 856–871, doi:10.1111/j.1574-6976.2011.00283.x (2011).
Leon-Sampedro, R., Novais, C., Peixe, L., Baquero, F. & Coque, T. M. Diversity and Evolution of the Tn5801-tet(M)-Like Integrative and Conjugative Elements among Enterococcus, Streptococcus, and Staphylococcus. Antimicrob Agents Chemother 60, 1736–1746, doi:10.1128/AAC.01864-15 (2016).
Munita, J. M., Bayer, A. S. & Arias, C. A. Evolving resistance among Gram-positive pathogens. Clin Infect Dis 61(Suppl 2), S48–57, doi:10.1093/cid/civ523 (2015).
Muraih, J. K., Pearson, A., Silverman, J. & Palmer, M. Oligomerization of daptomycin on membranes. Biochim Biophys Acta 1808, 1154–1160, doi:10.1016/j.bbamem.2011.01.001 (2011).
Silverman, J. A., Perlmutter, N. G. & Shapiro, H. M. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother 47, 2538–2544 (2003).
Nallapareddy, S. R., Singh, K. V. & Murray, B. E. Contribution of the collagen adhesin Acm to pathogenesis of Enterococcus faecium in experimental endocarditis. Infect Immun 76, 4120–4128, doi:10.1128/IAI.00376-08 (2008).
Hendrickx, A. P. et al. SgrA, a nidogen-binding LPXTG surface adhesin implicated in biofilm formation, and EcbA, a collagen binding MSCRAMM, are two novel adhesins of hospital-acquired Enterococcus faecium. Infect Immun 77, 5097–5106, doi:10.1128/IAI.00275-09 (2009).
Top, J. et al. The Enterococcus faecium enterococcal biofilm regulator, EbrB, regulates the esp operon and is implicated in biofilm formation and intestinal colonization. PloS one 8, e65224, doi:10.1371/journal.pone.0065224 (2013).
Ali, L. et al. Identification and functional characterization of the putative polysaccharide biosynthesis protein (CapD) of Enterococcus faecium U0317. Infect Genet Evol 37, 215–224, doi:10.1016/j.meegid.2015.11.020 (2016).
Wolska, K. I., Grudniak, A. M., Rudnicka, Z. & Markowska, K. Genetic control of bacterial biofilms. J Appl Genet 57, 225–238, doi:10.1007/s13353-015-0309-2 (2016).
Svensson, S. L. & Sharma, C. M. Small RNAs in Bacterial Virulence and Communication. Microbiol Spectr 4, doi:10.1128/microbiolspec.VMBF-0028-2015 (2016).
Bouloc, P. & Repoila, F. Fresh layers of RNA-mediated regulation in Gram-positive bacteria. Curr Opin Microbiol 30, 30–35, doi:10.1016/j.mib.2015.12.008 (2016).
Galperin, M. Y. et al. Expanded microbial genome coverage and improved protein family annotation in the COG database. Nucleic Acids Res 43, D261–9, doi:10.1093/nar/gku1223 (2015).
Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26, 589–595, doi:10.1093/bioinformatics/btp698 (2010).
Nawrocki, E. P. et al. Rfam 12.0: updates to the RNA families database. Nucleic Acids Research. doi:10.1093/nar/gku1063 (2014).
Nawrocki, E. P. & Eddy, S. R. Infernal 1.1: 100-fold faster RNA homology searches. Bioinformatics 29, 2933–2935, doi:10.1093/bioinformatics/btt509 (2013).
Acknowledgements
We warmly thank Michel Auzou for his excellent technical assistance and the eBio IFB platform for bioinformatics support (ANR-11-INBS-0013). This work was supported by a grant from the Ministère de l’Enseignement Supérieur et de la Recherche to EA4655, Université de Caen Normandie, France. JB is a recipient of a fellowship from the Direction Générale pour l’Armement and the Conseil Régional de Bretagne. MS was supported in part by the region Bretagne grant SAD SARS_2 9181. YA was supported by a Marie Curie International Incoming Fellowship (project 621959-SarHyb) within the 7th European Community Framework Program. BF is a recipient from the Agence Nationale pour la Recherche (grant sRNA-fit).
Author information
Authors and Affiliations
Contributions
V.C. and B.F. conceived and designed the experiments; C.S., M.S., J.B., M.C. and F.G. performed the experiments; M.S., Y.A., P.M., and V.C. performed the bioinformatics studies; C.S., Y.A., M.S., M.C., F.G., V.C. and B.F. analysed the data; M.S., V.C. and B.F. contributed with reagents/materials/analysis tools; C.S., Y.A., M.S., V.C. and B.F. wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Accession codes: The RNA-seq data generated during this study were deposited into GEO under accession number GSE94924.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sinel, C., Augagneur, Y., Sassi, M. et al. Small RNAs in vancomycin-resistant Enterococcus faecium involved in daptomycin response and resistance. Sci Rep 7, 11067 (2017). https://doi.org/10.1038/s41598-017-11265-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-11265-2
This article is cited by
-
Distinct expression profiles of regulatory RNAs in the response to biocides in Staphylococcus aureus and Enterococcus faecium
Scientific Reports (2021)
-
Identification of the general stress stimulon related to colonization in Enterococcus faecalis
Archives of Microbiology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.