Abstract
Aryl hydrocarbon receptor (AhR) ligands are important for gastrointestinal health and play a role in gut inflammation and the induction of T regulatory cells, and the short chain fatty acids (SCFAs) butyrate, propionate and acetate also induce similar protective responses. Initial studies with butyrate demonstrated that this compound significantly increased expression of Ah-responsive genes such as Cyp1a1/CYP1A1 in YAMC mouse colonocytes and Caco-2 human colon cancer cell lines. Butyrate synergistically enhanced AhR ligand-induced Cyp1a1/CYP1A1 in these cells with comparable enhancement being observed for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and also microbiota-derived AhR ligands tryptamine, indole and 1,4-dihydroxy-2-naphthoic acid (DHNA). The effects of butyrate on enhancing induction of Cyp1b1/CYP1B1, AhR repressor (Ahrr/AhRR) and TCDD-inducible poly(ADP-ribose)polymerase (Tiparp/TiPARP) by AhR ligands were gene- and cell context-dependent with the Caco-2 cells being the most responsive cell line. Like butyrate and propionate, the prototypical hydroxyamic acid-derived histone deacetylase (HDAC) inhibitors Panobinostat and Vorinostat also enhanced AhR ligand-mediated induction and this was accompanied by enhanced histone acetylation. Acetate also enhanced basal and ligand-inducible Ah responsiveness and histone acetylation, demonstrating that acetate was an HDAC inhibitor. These results demonstrate SCFA-AhR ligand interactions in YAMC and Caco-2 cells where SCFAs synergistically enhance basal and ligand-induced expression of AhR-responsive genes.
Similar content being viewed by others
Introduction
The aryl hydrocarbon receptor (AhR) was initially identified as the hepatic intracellular protein that bound with high affinity to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a highly toxic industrial by-product1. The AhR partners with the AhR nuclear translocator (Arnt) to form a nuclear heterodimer that binds with cis-acting xenobiotic response element (XREs) in target gene promoters to induce expression of Ah-responsive genes such as CYP1A1 2, 3. Although the AhR is an essential target for mediating the toxicity of TCDD and structurally-related compounds4, 5, there is increasing evidence that the AhR plays an essential role in maintaining tissue-specific homeostasis6,7,8,9. For example, in AhR knockout mice, there are multiple deficits including reproductive tract and cardiovascular problems and failure of closure of the ductis venosis in liver6,7,8,9. The AhR also plays an important role in regulating the immune functions, inflammation, gastrointestinal health, stem cells and cancer, and this receptor is now emerging as an important drug target [rev. in refs 10,11,12,13,14,15].
TCDD, structurally-related halogenated aromatics, and carcinogenic polynuclear aromatic hydrocarbons were the first compounds identified as AhR ligands; however, subsequent studies have identified structurally diverse synthetic and naturally occurring compounds as ligands for this receptor. These include industrial by-products, phytochemicals including flavonoids, indole-3-carbinol and related indole compounds, diverse pharmaceuticals, and possible endogenous ligands including formylindolino[2,3]b-carbazol (FICZ) [rev. in refs 15 and 16]. The identification of health-promoting compounds as AhR ligands has spurred research on the development and applications of AhR-active chemicals for chemotherapeutic applications.
The AhR plays a particularly important role in maintaining gastrointestinal health and several studies show that AhR expression in subsets of gut epithelial cells are important for bacterial resistance, gut inflammation and integrity and this has been associated with induction of T regulatory (Treg) cells, FoxP3 and interleukin-2217,18,19,20,21,22,23,24,25. Moreover, these same studies show that AhR-active microbiota-derived metabolites including tryptophan catabolites or exogenous AhR-active compounds play a role in maintaining intestinal integrity and bacterial resistance and inhibiting inflammation. Interestingly, short chain fatty acids (SCFAs) such as butyrate exhibit activities similar to that observed for AhR agonists including induction of Treg cells, anti-inflammatory activity and induction of interleukin 2226,27,28,29. Since microbiota simultaneously produce both AhR ligands and SCFAs, we used human Caco-2 anticancer cells and mouse YMAC colonocytes as models to investigate the interaction of SCFAs and AhR ligands and their effects on Ah-responsive gene expression. The results demonstrate that acetate, propionate and butyrate enhance AhR ligand-induced responses via their activities as histone deacetylase (HDAC) inhibitors; however, the effectiveness of these interactions are both gene- and cell context-dependent. Our data also show for the first time that one of the most abundant SCFAs, acetate, is also in HDAC inhibitor.
Materials and Methods
Cell lines, antibodies, and reagents
The young adult mouse colonic (YAMC) cell line and the AhR knockout cells (YAMC-AhR-KO) were described in our previous studies30. Cells were maintained in RPMI 1640 medium with 5% fetal bovine serum, 5 units/ml mouse interferon-γ (IF005) (EMD Millipore, MA), 0.1% ITS “−” minus (insulin, transferrin, selenium) (41-400-045) (Life Technologies, Grand Island, NY) at 33 °C (permissive conditions) and experiments were carried out at 37 °C (nonpermissive conditions). Caco-2 human colon cancer cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM) nutrient mixture supplemented with 20% fetal bovine serum (FBS), 10 ml/L 100X MEM non-essential amino acid solution (Gibco), and 10 ml/L 100X antibiotic/antimycotic solution (Sigma-Aldrich, St. Louis, MO) at 37 °C in the presence of 5% CO2. β-Actin antibody was purchased from Sigma-Aldrich, and mouse CYP1A1 antibody was kindly provided by the late Dr. Paul Thomas (Rutgers University) and Dr. B. Moorthy (Baylor College of Medicine, Houston). Antibodies to human CYP1A1 and AhR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Acetyl-H3K9/K14, acetyl-H3K27, and acetyl-H4K8 antibodies were purchased from Cell Signaling Technology (Danvers, MA). Sodium butyrate, sodium propionate, and sodium acetate were purchased from Sigma-Aldrich, and Panobinstat, Entinostat, Vorinostat were purchased from LC Laboratories (Woburn, MA). CH223191 was purchased from TOCRIS Bioscience (Minneapolis, MN).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using ChIP-IT Express Magnetic Chromatin Immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. YAMC and Caco-2 cells (1 × 107 cells) were treated with sodium butyrate overnight; 10 nM TCDD was added and after 2 hr, cells were fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to desired chromatin length (~200–1500-bp). The sonicated chromatin was immunoprecipitated with primary antibodies and protein A-conjugated magnetic beads at 4 °C for 8 hr. The magnetic beads were extensively washed, protein-DNA crosslinks were reversed and eluted, and DNA was prepared by proteinase K digestion followed by PCR amplification. The mouse Cyp1a1 primers were 5′-CAG GAG AGC TGG CCC TTT A-3′ (sense) and 5′-TAA GCC TGC TC ATC CTG TG-3′ (antisense), and subsequently amplified by targeting a 215-bp region of mouse Cyp1a1 promoter, which contained AhR-binding sequences. The human CYP1A1 primers were 5′-TCA ATC AAG AGG CGC GAA CCT C-3′ (sense), and 5′-CTA CAG CCT ACC AGG ACT CG-3′ (antisense), and then amplified a 203-bp region of human CYP1A1 promoter which contained the AhR binding sequences. PCR products were resolved on a 2% agarose gel in the presence of ethidium bromide.
Quantitative real-time PCR
cDNA was prepared from the total RNA of cells using High Capacity RNA-to-cDNA Kit (Applied Biosystems, Foster City, CA). Each PCR was carried out in triplicate in a 20 μL volume using SYBR Green Q-PCR Master mix (GenDEPOT, Katy, TX) for 1 min at 95 °C for initial denaturing, followed by 40 cycles of 95 °C for 15 sec and 60 °C for 1 min in the Bio-Rad iCycler (MyiQ™2) real-time PCR System. The comparative CT method was used for relative quantitation of samples. Values for each gene were normalized to expression levels of TATA-binding protein (TBP). The sequences of the primers used for real-time PCR are summarized in Supplementary Table S1.
Western blot analysis
Cells (3 × 105) were plated in six-well plates in DMEM media containing 2.5% FBS for 24 hr and then treated with different concentrations of the compounds. Cell lysates were prepared in lysis buffer containing 50 mM HEPES, 0.5 M NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, and 1% Triton-X-100, each 10 μL/ml protease and phosphatase inhibitor cocktail (GenDEPOT) and 1% NP-40. The cells were disrupted and extracted at 4 °C for 30 min and after centrifugation, the supernatant was obtained as the cell lysate. Protein concentrations were measured using the Bio-Rad protein assay. Aliquots of cellular proteins were electrophoresed on 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to a PVDF membrane (Bio-Rad, Hercules, CA). The membrane was allowed to react with a specific antibody, and detection of specific proteins was carried out by enhanced chemiluminescence. Loading differences were normalized using a polyclonal β-actin antibody.
Animals and compounds administration
Mice (C57BL6/J) were housed in the Texas A&M University animal facility with a 12-hr light/dark cycle and constant temperature (23–25 °C). The mice had free access to water and diet. All procedures were performed in accordance with National Institutes of Health guidelines for the care and use of animals and were approved by the institutional animal care and use committee at Texas A&M University. For experiments involving butyrate and/or DHNA treatment, mice (8–10 weeks of age) were gavaged once per day with butyrate (1 g/kg in water) and/or 1,4-dihydroxy 2-naphthoic acid (DHNA, 20 mg/kg in water) for 3 days and killed 6 hr after the last treatment.
Statistics
All of the experiments were repeated a minimum of three times. The data are expressed as the means ± SD. Statistical significance was analyzed using either Unpaired-Student’s t-test (two-tailed) or analysis of variance (ANOVA) test. A P value of less than 0.05 was considered statistically significant.
Results
Butyrate enhances basal and TCDD-induced Ah-responsive gene expression
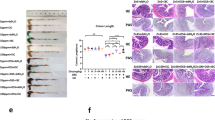
Sodium butyrate is a major microbiota-derived metabolite and potent HDAC inhibitor and there are conflicting reports showing that butyrate enhances31 or does not affect32 basal or AhR ligand-induced CYP1A1/CYP1A1-promoter activity. Treatment of YAMC and Caco-2 cells with 1–10 mM butyrate had minimal effects on AhR mRNA levels in YAMC cells but increased expression in Caco-2 cells (Fig. 1A). Butyrate alone induced two Ah-responsive genes, Cyp1a1/CYP1A1 (Fig. 1B) and Cyp1b1/CYP1B1 (Fig. 1C), in both YAMC and Caco-2 cells and the fold and maximal induction responses were generally higher for Cyp1a1/CYP1A1. Butyrate also significantly induced AhRR and Tiparp gene expression in both cell lines (Fig. 1D and E) and there was some cell context- and concentration-dependent variability in these responses. Using Cyp1a1/CYP1A1 as a model, butyrate-induced gene expression was inhibited by the AhR antagonist CH223191 (Fig. 1F) and we observed that butyrate induction of Cyp1a1 was also blocked in YAMC-AhR-KO cells (Fig. 1G) in which the AhR was knocked out via CRISPR/Cas9 as described30. Thus, butyrate induces Ah-responsive genes and this response is AhR-dependent; however, as indicated in subsequent studies (Fig. 2), the magnitude of the Cyp1a1 response was >5% of the induction response observed for TCDD.
Butyrate modulates expression of Ah-responsive genes in YAMC and Caco-2 cells. Cells were treated with DMSO or 1–10 mM butyrate for 24 hr, and expression of AhR mRNA (A) and protein (B) were determined by real time PCR and western blots, respectively. Cells were treated with DMSO and 1–10 mM butyrate for 24 hr, and levels of Cyp1a1/CYP1A1 (C), Cyp1b1/CYP1B1 (D), Ahrr/AhRR (D) and Tiparp/TiPARP (F) mRNAs were determined by real time PCR. (G) Cells were treated with butyrate alone or in combination with CH223191, and Cyp1a1/CYP1A1 mRNA was determined by real time PCR. (H) YAMC-AhR-KO cells were treated with butyrate, and Cyp1a1 mRNA levels were determined by real time PCR. Results are expressed as means ± SE (3 replicated determination), and significantly (p < 0.05) induced (*) and inhibited (**) responses are indicated.
Butyrate enhances TCDD-induced gene expression YAMC and Caco-2 cells. Cells were treated with DMSO, TCDD and TCDD plus 5 mM butyrate, and effects on Cyp1a1/CYP1A1 (A), Cyp1b1/CYP1B1 (B), Ahrr/AhRR (C) and Tiparp/TiPARP (D) mRNA levels were determined by real time PCR. (E) Cells were treated with DMSO, TCDD, butyrate and their combination for 24 hr, and whole cell lysates were analyzed by western blots. (F) Cells were treated with DMSO, butyrate, TCDD and TCDD plus butyrate and also in combination with CH223191, and Cyp1a1/CYP1A1 mRNA levels were determined by real time PCR. (G) Cells were treated with DMSO, 5 mM butyrate, different concentrations of TCDD alone and TCDD plus butyrate, and Cyp1a1/CYP1A1 mRNA levels were determined by real time PCR. With the exception of AhRR in Caco-2 cells, TCDD significantly induced all other Ah-responsive genes, and significant (p < 0.05) enhancement by butyrate is indicated (*). Results are expressed as means ± SE for at least 3 separate determinations for each treatment group. Significant (p < 0.05) inhibition by CH223191 is indicated (**).
We also investigated the effects of butyrate on TCDD-induced gene expression in YAMC and Caco-2 cells and Fig. 2A illustrates that butyrate enhanced TCDD-induced Cyp1a1/CYP1A1 gene expression in YAMC and Caco-2 cells approximately 3- to 4-fold. However, the fold enhancement of other Ah-responsive genes was both cell context- and response-dependent. In YAMC cells, induction of Cyp1b1/CYP1B1 (Fig. 2B), AhRR (Fig. 2C) and TiPARP (Fig. 2D) by TCDD was minimally or not enhanced after cotreatment with butyrate, whereas highly significant enhancement was observed for all genes in Caco-2 cells. Interestingly, AhRR was not induced by TCDD in Caco-2 cells but in combination with butyrate, there was a >6-fold induction response. TCDD (10 nM) alone or in combination with 5 mM butyrate induced expression of Cyp1a1 protein and butyrate significantly enhanced this response in YAMC cells, whereas minimal enhancement was observed in Caco-2 cells (Fig. 2E). Butyrate increased expression of AhR protein in both cell lines and TCDD alone or in combination with butyrate decreased expression of AhR protein. This effect is commonly observed in most cell lines due to proteasome-dependent degradation of the AhR. Results in Fig. 2F show that the AhR antagonist CH223191 inhibited both TCDD- and TCDD plus butyrate-induced Cyp1a1/CYP1A1 expression in both YAMC and Caco-2 cells. We also investigated butyrate (5 mM) enhancement of 0.001–10 nM TCDD effects in YAMC and Caco-2 cells (Fig. 2G) and show that butyrate comparably enhanced TCDD-induced Cyp1a1 gene expression in both cell lines using high and low concentrations of TCDD.
SCFAs enhance ligand-activated Ah-responsive genes
Previous studies show that tryptophan metabolites tryptamine, indole and 1,4-dihydroxy-2-naphthoic acid (DHNA) are derived from microbiota and exhibit AhR agonist/antagonist activities in colon and other cell lines30, 33,34,35,36. Therefore, we investigated interactions of 5 mM butyrate with indole, tryptamine and DHNA and expression of AhR (Fig. 3A) and the Ah-responsive genes Cyp1a1/CYP1A1 (Fig. 3B), Cyp1b1/CYP1B1 (Fig. 3C), Ahrr/AhRR (Fig. 3D) and Tiparp/TiPARP (Fig. 3E) in YAMC and Caco-2 cells. Minimal interactions (<2-fold) were observed for AhR expression; however, the effects of butyrate on DHNA and the relative weak AhR agonists tryptamine and indole were comparable to those observed for TCDD (Fig. 2). Butyrate enhanced induction of Cyp1a1/CYP1A1 by indole, tryptamine and DHNA in YAMC and Caco-2 cells, and butyrate also enhanced induction of CYP1A1, AhRR, and TiPARP by the microbiota-derived metabolites only in Caco-2 cells. We also carried out an in vivo pilot study by treating C57BL/6 mice with butyrate (1 g/kg/d) and DHNA (20 mg/kg/d) for 3 days and observed minimal induction of Cyp1a1 or Cyp1a2 by the compounds alone but in combination, there was induction of Cyp1a1 (liver, colon) and Cyp1a2 (liver) (Suppl. Fig. S1).
Butyrate enhances AhR ligand-induced gene expression in YAMC and Caco-2 cells. Cells were treated with DMSO, different AhR ligands alone or in combination with 5 mM butyrate and effects on expression of AhR (A), Cyp1a1/CYP1A1 (B), Cyp1b1/CYP1B1 (C), Ahrr/AhRR (D) and Tiparp/TiPARP (E) mRNA levels were determined by real time PCR. With the exception of AhRR mRNA, AhR ligands significantly induced all other Ah-responsive genes, and significant (p < 0.05) enhancement by butyrate is indicated (*). Results are expressed as means ± SE for at least 3 separate determinations for each treatment group.
Previous studies reported that the SCFAs butyrate and propionate, but not acetate, were HDAC inhibitors26, 37, 38 and therefore using Cyp1a1 and Cyp1b1 as Ah-responsive genes, we further investigated effects of propionate and acetate on Ah-responsive gene expression. Like butyrate, both propionate and acetate increased expression of Cyp1a1/CYP1A1 in YAMC and Caco-2 cells and similar results were observed for expression of Cyp1b1/CYP1B1 in both cell lines (Fig. 4A). Propionate and acetate also significantly enhanced TCDD-induced Cyp1a1 and CYP1A1 expression in YAMC and Caco-2 cells (Fig. 4B, top), respectively, and similar results were observed for butyrate (Fig. 2). In YAMC cells treated with TCDD, cotreatment with propionate or acetate had minimal effects on Cyp1b1 mRNA levels, whereas both SCFAs significantly enhanced TCDD-induced CYP1B1 expression in Caco-2 cells (Fig. 4B, bottom). Results summarized in Fig. 4C show that propionate and acetate enhanced tryptophan-, indole- and DHNA-induced Cyp1a1 (YAMC and Caco-2 cells) and CYP1B1 (Caco-2 cells). Thus, acetate, propionate and butyrate, the major SCFAs generated by microbial fermentation of fiber in the gut, enhanced basal and AhR ligand-induced expression of Cyp1a1/CYP1A1in both Caco-2 and YAMC cells and enhancement of ligand-induced Cyp1b1/CYP1B1 was primarily observed in Caco-2 cells.
Acetate and propionate enhances Cyp1a1/CYP1A1 expression in YAMC and Caco-2 cells. (A) Cells were treated with DMSO and different concentrations of acetate or propionate and expression of Cyp1a1/CYP1A1 and Cyp1b1/CYP1B1 mRNA levels was determined by real time PCR. (B) YAMA or Caco-2 cells were treated with DMSO, 10 nM TCDD alone and in combination with different concentrations of acetate or propionate, and expression of Cyp1a1/CYP1A1 (C) and Cyp1b1/CYP1B1 (D) mRNA levels was determined by real time PCR. (C) YAMC or Caco-2 cells were treated with DMSO, propionate and acetate alone and in combination with microbiota-derived AhR ligand, and Cyp1a1/CYP1A1 and Cyp1b1/CYP1B1 mRNA levels were determined by real time PCR. Significant (p < 0.05) induction of Cyps/CYPs by acetate or propionate alone and propionate/acetate-enhanced induction of Cyps/CYPs by AhR ligand is indicated (*). Results are expressed as means ± SE for at least 3 separate experiments for each treatment group.
HDAC inhibitors enhance expression of Ah-responsive genes
We also investigated the effects of two synthetic HDAC inhibitors Panobinostat and Vorinostat on TCDD-induced Ah-responsive genes in YAMC and Caco-2 cells. In YAMC cells, Panobinostat and Vorinostat enhanced TCDD-induced Cyp1a1/CYP1A1 gene expression (Fig. 5A) but at the concentration used they were less effective than butyrate. For TCDD-induced responses such as Cyp1b1/CYP1B1 (Fig. 5B), Ahrr (Fig. 5C) and Tiparp (Fig. 5D) where butyrate had minimal enhancing activity, similar results were observed for Panobinostat and Vorinastat, and the former compound decreased TCDD-induced Cyp1b1 and AhRR in YAMC cells. In contrast, like the SCFAs, Panobinostat and Vorinostat enhanced TCDD-induced CYP1B1, AhRR and TiPARP and with the exception of CYP1B1, the magnitude of enhanced responses for hydroxamic acid-derived synthetic HDAC inhibitors and SCFAs was similar.
HDAC inhibitors enhance TCDD-induced gene expression in YAMC and Caco-2 cells. Cells were treated with DMSO alone, TCDD alone, and TCDD in combination with different HDAC inhibitors, and Cyp1a1/CYP1A1 (A), Cyp1b1/CYP1B1 (B), Ahrr/AhRR (C) and Tiparp/TiPARP (D) mRNA levels were determined by real time PCR. Significant (p < 0.05) enhancement of TCDD-induced gene expression by HDAC inhibitors is indicated (*). Results are expressed as means ± SE for 3 separate determinations for each treatment group.
SCFAs enhance histone acetylation and recruitment of the AhR to Cyp1a1/CYP1A1 promoters
After treating YAMC cells with 10 nM TCDD alone, butyrate, Panobinostat and Vorinostat alone, and in combination with TCDD for 18 hr, we examined expression of the AhR and acetylated histones (H3K9/K14, H3K27 and H4K8) by western blots. TCDD alone decreased AhR expression but did not affect histone acetylation in YAMC cells (Fig. 6A), whereas the HDAC inhibitors alone and in combination with TCDD increased acetylation of H3K9/K14, H3K27 and H4K8. Similar results were observed in Caco-2 cells (Fig. 6B), demonstrating that the HDAC inhibitors induced overall histone acetylation in both cell lines. We also investigated butyrate-TCDD/AhR interactions with an Ah-responsive (DRE-containing) region of the Cyp1a1 promoter in YAMC cells in a ChIP assay and observed that TCDD, butyrate and their combination induced recruitment of the AhR to the promoter and cotreatment did not further enhance AhR recruitment (Fig. 6C). Binding of pol II to the promoter was also enhanced by the different treatments and histone acetylation was observed in both the untreated and treated cells. In contrast, histone acetylation associated with untreated Caco-2 cells was not observed (Fig. 6D) but was induced by TCDD, butyrate and butyrate plus TCDD, and similar results were observed for treatment-related recruitment of the AhR and Pol II to the Ah-responsive region of the CYP1A1 promoter. Thus, there were major differences in histone acetylation of the Cyp1a1/CYP1A1 promoters in untreated YAMC and Caco-2 cells and this may account for the higher inducibility of this gene in YAMC cells.
Effects of SCFAs and HDAC inhibitors on histone acetylation in ChIP assays. YAMC (A) and Caco-2 (B) cells were treated with DMSO, 10 nM TCDD alone or in combination with SCFAs and HDAC inhibitors for 24 hr, and whole cell lysates were analyzed by western blots. YAMC (C) and Caco-2 (D) cells were treated with DMSO, 10 nM TCDD and 5 mM butyrate alone or in combination for 4 hr, and ChIP assays were carried out as outlined in the Materials and Methods to detect interactions with the XRE-containing regions of the Cyp1a1 (mouse) and CYP1A1 (human) promoters.
Discussion
The complex human gut microbiome plays a pivotal role in the health of the gastrointestinal tract and multiple distal organs and tissues39. These interactions are dependent on the diet and other factors that influence the composition of the microbiome and microbial metabolites that in turn directly affect the host through initial interactions with multiple targets on intestinal cells39,40,41. For example, humans on a plant-based vs. animal-based diet exhibited significant differences in bacterial taxonomic groups and this was particularly evident for the genus Prevotella which is induced by dietary fiber but is reduced in the animal-based diet42. Moreover, dietary-induced changes in the microbiome also resulted in changes in microbiota-derived metabolites in which SCFAs such as acetate and butyrate were higher in plant-based diets, whereas isovalerate and isobutyrate were higher in animal-based diets42. Plant-based diets are relatively high in fiber which induces commensal bacteria resulting in higher levels of SCFAs which in turn exhibit antiinflammatory activities26,27,28,29. Enhanced tryptophan metabolism by Lactobacilli induces formation of indole-derived Ah receptor ligands19 which also exhibit antiinflammatory activities and resistance to infection18,19,20,21,22,23,24,25. Moreover, tryptophan is degraded to form niacin, and both niacin and butyrate suppress intestinal inflammation by activation of the common receptor Gpr109a43, 44.
The effects of butyrate and other HDAC inhibitors on basal and ligand-induced Ah-responsive genes is highly variable; one study showed that butyrate enhanced an AhR-responsive gene promoter in vitro 31 and butyrate also enhanced expression of Ah-responsive genes in the mouse intestine45, whereas another study indicated that HDAC1 inhibition was insufficient to induce Cyp1a132. The effects of HDAC inhibitors on ligand-induced CYP1A1 resulted in decreased expression in aerodigestive epithelial cells and increased expression in colon epithelial cells and fibroblasts46,47,48. This study extensively investigated AhR agonist-SCFA interactions on Ah-responsive gene expression in vitro using Caco-2 and YAMC cells which are responsive to both TCDD, DHNA and tryptophan metabolites30, 33, 34.
Among the SCFAs butyrate has been extensively identified as an HDAC inhibitor. Thus, we initially examined the effects of butyrate alone and in combination with TCDD and three microbiota-derived AhR ligands, indole, tryptamine and DHNA on expression of four Ah-responsive genes (Cyp1a1, Cyp1b1, AhRR and TiPARP). Butyrate induced Cyp1a1/CYP1A1 and Cyp1b1/CYP1B1 mRNA levels in both YAMC and Caco-2 cells (Fig. 1) and the latter response was accompanied by enhanced histone acetylation (H3K9/14, H3K27 and H4K8) in both cell lines (Fig. 6). Moreover, in ChIP assays, we also observed that treatment with butyrate recruited the AhR and pol II to the Cyp1a1 promoter in both cell lines and enhanced H3K47Ac and H3K9Ac on the CYP1A1 promoter only in Caco-2 cells (Fig. 6A–D), whereas the histone marks were observed on the Cyp1a1 promoter in untreated YAMC cells. These results are consistent with an induction (Cyp1a1) response by butyrate due to its activity as an HDAC inhibitor and similar results were observed for hydroxamic acid-derived HDAC inhibitors Vorinostat and Panobinostat (Fig. 5). Butyrate also significantly enhanced AhRR and TiPARP gene expression in Caco-2 cells but the magnitude of these responses (1.5- to 3-fold) were lower than observed for Cyp1a1. The effects of butyrate alone on Ah-responsive genes in YAMC and Caco-2 cells was also observed for propionate, and this is consistent with previous studies showing that like butyrate, propionate is also an HDAC inhibitor26, 37, 38. The highest concentration SCFA produced by microorganisms in the gut is usually acetate26 and in this study, we observed that acetate concentrations >20 mM also enhanced expression of Ah-responsive genes in YAMC and Caco-2 cells (Fig. 4). Acetate also enhanced overall histone acetylation in these cell lines (Fig. 6), demonstrating for the first time that acetate is also an HDAC inhibitor and thus, contributes to the overall HDAC inhibitory activity of SCFAs.
We also observed that the effects of all three SCFAs on AhR ligand-induced gene expression were similar for TCDD, tryptamine, indole and DHNA but were both cell context- and gene-dependent. AhR ligand-induced Cyp1a1/CYP1A1 was enhanced by SCFAs in both YAMC and Caco-2 and similar results were observed for the hydroxamic acid-derived HDAC inhibitors Panobinostat and Vorinostat. The HDAC inhibitors enhanced AhR ligand-mediated induction of CYP1B1, AhRR and TiPARP in Caco-2 cells, whereas in YAMC cells, the enhancement was minimal to non-detectable and this was similar to that observed for SCFAs.
The reasons for the gene- and cell-specific interactions of SCFAs and AhR ligands and their impacts on intestinal health are unknown and are currently under investigation. However, since recent reports indicate that both the AhR and Cyp1a1 are necessary for maintaining health-promoting levels of microbiota-derived natural AhR ligands49,50,51, it is possible that SCFAs also play a role in maintaining intestinal AhR and enhanced Cyp1a1 activity in vivo. In a pilot study, we showed that treatment of C57BL/6 mice with 1 g/kg/d of butyrate and 20 mg/kg/d of DHNA as previously described45, 52 did not significantly induce liver or colon Cyp1a1 or Cyp1a2 (Suppl. Fig. S1). The reason for these differences are unclear; however, it should be noted the magnitude of the reported induction responses was small45, 52. In contrast, we observed induction of Cyp1a1 in the liver and colon in animals cotreated with DHNA plus butyrate, and over 50-fold induction was observed in liver with a large standard error due to mouse variability. DHNA plus butyrate also significantly induced Cyp1a2 in liver but not colon and this is consistent with a previous report indicating that Cyp1a2 was not inducible in the mouse colon53. We are currently refining methods for enhancing SCFA production and future studies will investigate the role of SCFAs on AhR ligand-dependent activation of IL-22 and Tregs and inhibition of intestinal inflammation in mouse models.
References
Poland, A., Glover, E. & Kende, A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J. Biol. Chem. 251, 4936–4946 (1976).
Hankinson, O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 35, 307–340 (1995).
Whitlock, J. P. Jr. Induction of cytochrome P4501A1. Annu. Rev. Pharmacol. Toxicol. 39, 103–125 (1999).
Fernandez-Salguero, P., Hilbert, D. M., Rudikoff, S., Ward, J. M. & Gonzalez, F. J. Aryl hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 140, 173–179 (1996).
Gonzalez, F. J. & Fernandez-Salguero, P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab. Dispos. 26, 1194–1198 (1998).
Lahvis, G. P. et al. The aryl hydrocarbon receptor is required for developmental closure of the ductus venosus in the neonatal mouse. Mol. Pharmacol. 67, 714–720 (2005).
Benedict, J. C., Lin, T. M., Loeffler, I. K., Peterson, R. E. & Flaws, J. A. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol. Sci. 56, 382–388 (2000).
Lund, A. K., Goens, M. B., Nunez, B. A. & Walker, M. K. Characterizing the role of endothelin-1 in the progression of cardiac hypertrophy in aryl hydrocarbon receptor (AhR) null mice. Toxicol. Appl. Pharmacol. 212, 127–135 (2006).
McMillan, B. J. & Bradfield, C. A. The aryl hydrocarbon receptor sans xenobiotics: endogenous function in genetic model systems. Mol. Pharmacol. 72, 487–498 (2007).
Marshall, N. B. & Kerkvliet, N. I. Dioxin and immune regulation: emerging role of aryl hydrocarbon receptor in the generation of regulatory T cells. Ann. N. Y. Acad. Sci. 1183, 25–37 (2010).
Hao, N. & Whitelaw, M. L. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 86, 561–570 (2013).
Stevens, E. A., Mezrich, J. D. & Bradfield, C. A. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 127, 299–311 (2009).
Busbee, P. B., Rouse, M., Nagarkatti, M. & Nagarkatti, P. S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 71, 353–369 (2013).
Esser, C. Biology and function of the aryl hydrocarbon receptor: report of an international and interdisciplinary conference. Arch. Toxicol. 86, 1323–1329 (2012).
Denison, M. S., Soshilov, A. A., He, G., DeGroot, D. E. & Zhao, B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 124, 1–22 (2011).
Safe, S., Chadalapaka, G. & Jutooru, I. In The Ah Receptor in Biology and Toxicology (ed. R. Pohjanvirta) Ch. 24, 331–342 (John Wiler & Sons, 2012).
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Monteleone, I. et al. Smad7 Knockdown Restores Aryl Hydrocarbon Receptor-mediated Protective Signals in the Gut. J Crohns Colitis 10, 670–677 (2016).
Zelante, T. et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 (2013).
Lee, J. S. et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151 (2012).
Gagliani, N. et al. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 (2015).
Monteleone, I. et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 141, 237–248, 248 e231 (2011).
Qiu, J. et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104 (2012).
Li, Y. et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 (2011).
Singh, N. P. et al. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6, e23522 (2011).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Bachmann, M., Meissner, C., Pfeilschifter, J. & Muhl, H. Cooperation between the bacterial-derived short-chain fatty acid butyrate and interleukin-22 detected in human Caco2 colon epithelial/carcinoma cells. Biofactors, In press (2016).
Fung, K. Y., Cosgrove, L., Lockett, T., Head, R. & Topping, D. L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 108, 820–831 (2012).
Segain, J. P. et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut 47, 397–403 (2000).
Cheng, Y. et al. Editor’s Highlight: Microbial-Derived 1,4-Dihydroxy-2-naphthoic Acid and Related Compounds as Aryl Hydrocarbon Receptor Agonists/Antagonists: Structure-Activity Relationships and Receptor Modeling. Toxicol. Sci. 155, 458–473 (2017).
Garrison, P. M., Rogers, J. M., Brackney, W. R. & Denison, M. S. Effects of histone deacetylase inhibitors on the Ah receptor gene promoter. Arch. Biochem. Biophys. 374, 161–171 (2000).
Schnekenburger, M., Peng, L. & Puga, A. HDAC1 bound to the Cyp1a1 promoter blocks histone acetylation associated with Ah receptor-mediated trans-activation. Biochim. Biophys. Acta 1769, 569–578 (2007).
Jin, U. H. et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 85, 777–788 (2014).
Cheng, Y. et al. Aryl Hydrocarbon Receptor Activity of Tryptophan Metabolites in Young Adult Mouse Colonocytes. Drug Metab. Dispos. 43, 1536–1543 (2015).
Hubbard, T. D., Murray, I. A. & Perdew, G. H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 43, 1522–1535 (2015).
Hubbard, T. D. et al. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5, 12689 (2015).
Kiefer, J., Beyer-Sehlmeyer, G. & Pool-Zobel, B. L. Mixtures of SCFA, composed according to physiologically available concentrations in the gut lumen, modulate histone acetylation in human HT29 colon cancer cells. Br. J. Nutr. 96, 803–810 (2006).
Waldecker, M., Kautenburger, T., Daumann, H., Busch, C. & Schrenk, D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J. Nutr. Biochem. 19, 587–593 (2008).
Rooks, M. G. & Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352 (2016).
Lukens, J. R. et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature 516, 246–249 (2014).
Thorburn, A. N., Macia, L. & Mackay, C. R. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40, 833–842 (2014).
David, L. A. et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563 (2014).
Digby, J. E. et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler. Thromb. Vasc. Biol. 32, 669–676 (2012).
Thangaraju, M. et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 69, 2826–2832 (2009).
Korecka, A. et al. Bidirectional communication between the aryl hydrocarbon receptor (AhR) and the microbiome tunes host metabolism. Biofilms and Microbiomes 2, 16014 (2016).
Kekatpure, V. D., Dannenberg, A. J. & Subbaramaiah, K. HDAC6 modulates Hsp90 chaperone activity and regulates activation of aryl hydrocarbon receptor signaling. J. Biol. Chem. 284, 7436–7445 (2009).
Zapletal, O. et al. Butyrate alters expression of cytochrome P450 1A1 and metabolism of benzo[a]pyrene via its histone deacetylase activity in colon epithelial cell models. Arch. Toxicol. (2016).
Haarmann-Stemmann, T. et al. Analysis of the transcriptional regulation and molecular function of the aryl hydrocarbon receptor repressor in human cell lines. Drug Metab. Dispos. 35, 2262–2269 (2007).
Schiering, C. et al. Feedback control of AHR signalling regulates intestinal immunity. Nature 542, 242–245 (2017).
Lamas, B. et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 (2016).
Murray, I. A., Nichols, R. G., Zhang, L., Patterson, A. D. & Perdew, G. H. Expression of the aryl hydrocarbon receptor contributes to the establishment of intestinal microbial community structure in mice. Sci. Rep. 6, 33969 (2016).
Fukumoto, S. et al. Identification of a probiotic bacteria-derived activator of the aryl hydrocarbon receptor that inhibits colitis. Immunol. Cell Biol. (2014).
Dey, A., Jones, J. E. & Nebert, D. W. Tissue- and cell type-specific expression of cytochrome P450 1A1 and cytochrome P450 1A2 mRNA in the mouse localized in situ hybridization. Biochem. Pharmacol. 58, 525–537 (1999).
Acknowledgements
This work was supported by National Institutes of Health [R01-ES025713 (R.S.C., A.J., S.S.), R35-CA197707 (R.S.C.) and R01-CA202697 (R.S.C., C.D.A., A.J., S.S.)]; Cancer Prevention Research Institute of Texas (R.S.C.); Texas AgriLife Research (R.S.C., S.S.); the Ray Nesbitt Chair Endowment (A.J.); and the Sid Kyle Chair Endowment (S.S.).
Author information
Authors and Affiliations
Contributions
Designed research (U.H.J., Y.C., R.S.C., S.S.); Performed research (U.H.J., Y.C., H.P., L.A.D., E.S.C., A.A. and E.A.W.); Contributed new reagents or analytic tools (L.A.D.); Analyzed data (U.H.J. and Y.C.); Wrote the paper (S.S., R.S.C., A.J. and C.D.A.). All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, UH., Cheng, Y., Park, H. et al. Short Chain Fatty Acids Enhance Aryl Hydrocarbon (Ah) Responsiveness in Mouse Colonocytes and Caco-2 Human Colon Cancer Cells. Sci Rep 7, 10163 (2017). https://doi.org/10.1038/s41598-017-10824-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10824-x
This article is cited by
-
Unlocking the secrets: exploring the influence of the aryl hydrocarbon receptor and microbiome on cancer development
Cellular & Molecular Biology Letters (2024)
-
Usage of ectoine as a cryoprotectant for cryopreservation of lactic acid bacteria
Folia Microbiologica (2024)
-
Effect of the gut microbiota and their metabolites on postoperative intestinal motility and its underlying mechanisms
Journal of Translational Medicine (2023)
-
Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: role of tryptophan metabolites generated by gut host-microbiota
Journal of Molecular Medicine (2023)
-
A mechanism-based understanding of altered drug pharmacokinetics by gut microbiota
Journal of Pharmaceutical Investigation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.