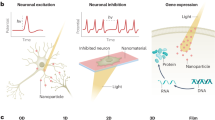
Abstract
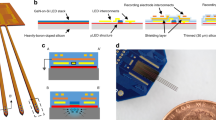
Optically controlled nongenetic neuromodulation represents a promising approach for the fundamental study of neural circuits and the clinical treatment of neurological disorders. Among the existing material candidates that can transduce light energy into biologically relevant cues, silicon (Si) is particularly advantageous due to its highly tunable electrical and optical properties, ease of fabrication into multiple forms, ability to absorb a broad spectrum of light, and biocompatibility. This protocol describes a rational design principle for Si-based structures, general procedures for material synthesis and device fabrication, a universal method for evaluating material photoresponses, detailed illustrations of all instrumentation used, and demonstrations of optically controlled nongenetic modulation of cellular calcium dynamics, neuronal excitability, neurotransmitter release from mouse brain slices, and brain activity in the mouse brain in vivo using the aforementioned Si materials. The entire procedure takes ~4–8 d in the hands of an experienced graduate student, depending on the specific biological targets. We anticipate that our approach can also be adapted in the future to study other systems, such as cardiovascular tissues and microbial communities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout










Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the original papers. Other supporting data are available upon reasonable request to the corresponding authors.
References
Ashkan, K., Rogers, P., Bergman, H. & Ughratdar, I. Insights into the mechanisms of deep brain stimulation. Nat. Rev. Neurol. 13, 548–554 (2017).
Famm, K., Litt, B., Tracey, K. J., Boyden, E. S. & Slaoui, M. Drug discovery: a jump-start for electroceuticals. Nature 496, 159–161 (2013).
Wichmann, T. & Delong, M. R. Deep brain stimulation for neurologic and neuropsychiatric disorders. Neuron 52, 197–204 (2006).
Fisher, R. S. & Velasco, A. L. Electrical brain stimulation for epilepsy. Nat. Rev. Neurol. 10, 261–270 (2014).
Jefferys, J. G. Nonsynaptic modulation of neuronal activity in the brain: electric currents and extracellular ions. Physiol. Rev. 75, 689–723 (1995).
Fregni, F. & Pascual-Leone, A. Technology insight: noninvasive brain stimulation in neurology—perspectives on the therapeutic potential of rTMS and tDCS. Nat. Clin. Pract. Neurol. 3, 383–393 (2007).
Perlmutter, J. S. & Mink, J. W. Deep brain stimulation. Annu. Rev. Neurosci. 29, 229–257 (2006).
Kringelbach, M. L., Jenkinson, N., Owen, S. L. & Aziz, T. Z. Translational principles of deep brain stimulation. Nat. Rev. Neurosci. 8, 623–635 (2007).
Chen, R., Canales, A. & Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2, 16093 (2017).
Lacour, S. P., Courtine, G. & Guck, J. Materials and technologies for soft implantable neuroprostheses. Nat. Rev. Mater. 1, 16063 (2016).
Salatino, J. W., Ludwig, K. A., Kozai, T. D. Y. & Purcell, E. K. Glial responses to implanted electrodes in the brain. Nat. Biomed. Eng. 1, 862–877 (2017).
Jeong, J. W. et al. Soft materials in neuroengineering for hard problems in neuroscience. Neuron 86, 175–186 (2015).
Minev, I. R. et al. Electronic dura mater for long-term multimodal neural interfaces. Science 347, 159–163 (2015).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Zhang, F. et al. The microbial opsin family of optogenetic tools. Cell 147, 1446–1457 (2011).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Bareket, L. et al. Semiconductor nanorod-carbon nanotube biomimetic films for wire-free photostimulation of blind retinas. Nano Lett. 14, 6685–6692 (2014).
Carvalho-de-Souza, J. L. et al. Photosensitivity of neurons enabled by cell-targeted gold nanoparticles. Neuron 86, 207–217 (2015).
Ghezzi, D. et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 7, 400–406 (2013).
Maya-Vetencourt, J. F. et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 16, 681–689 (2017).
Kim, D. H. et al. Stretchable and foldable silicon integrated circuits. Science 320, 507–511 (2008).
Patolsky, F. et al. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 313, 1100–1104 (2006).
Tian, B. et al. Three-dimensional, flexible nanoscale field-effect transistors as localized bioprobes. Science 329, 830–834 (2010).
Tian, B. et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 11, 986–994 (2012).
Jiang, Y. & Tian, B. Inorganic semiconductor biointerfaces. Nat. Rev. Mater. 3, 473–490 (2018).
Wang, W., Wu, S., Reinhardt, K., Lu, Y. & Chen, S. Broadband light absorption enhancement in thin-film silicon solar cells. Nano Lett. 10, 2012–2018 (2010).
Wang, K. X., Yu, Z., Liu, V., Cui, Y. & Fan, S. Absorption enhancement in ultrathin crystalline silicon solar cells with antireflection and light-trapping nanocone gratings. Nano Lett. 12, 1616–1619 (2012).
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Yun, S. H. & Kwok, S. J. J. Light in diagnosis, therapy and surgery. Nat. Biomed. Eng. 1, 0008 (2017).
Tian, B. et al. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 449, 885–889 (2007).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Hochbaum, A. I. et al. Enhanced thermoelectric performance of rough silicon nanowires. Nature 451, 163–167 (2008).
Roder, P. B., Smith, B. E., Davis, E. J. & Pauzauskie, P. J. Photothermal heating of nanowires. J. Phys. Chem. C 118, 1407–1416 (2014).
Jiang, Y. et al. Heterogeneous silicon mesostructures for lipid-supported bioelectric interfaces. Nat. Mater. 15, 1023–1030 (2016).
Parameswaran, R. et al. Photoelectrochemical modulation of neuronal activity with free-standing coaxial silicon nanowires. Nat. Nanotechnol. 13, 260–266 (2018).
Jiang, Y. et al. Rational design of silicon structures for optically controlled multiscale biointerfaces. Nat. Biomed. Eng. 2, 508–521 (2018).
Verkhratsky, A., Krishtal, O. A. & Petersen, O. H. From Galvani to patch clamp: the development of electrophysiology. Pflügers Arch. 453, 233–247 (2006).
Sakmann, B. & Neher, E. Single-Channel Recording 2nd edn (Springer, New York, 1995).
Hai, A., Shappir, J. & Spira, M. E. Long-term, multisite, parallel, in-cell recording and stimulation by an array of extracellular microelectrodes. J. Neurophysiol. 104, 559–568 (2010).
Spira, M. E. & Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 8, 83–94 (2013).
Robinson, J. T. et al. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol. 7, 180–184 (2012).
Ghezzi, D. et al. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2, 166 (2011).
Chen, R., Romero, G., Christiansen, M. G., Mohr, A. & Anikeeva, P. Wireless magnetothermal deep brain stimulation. Science 347, 1477–1480 (2015).
Dobson, J. Remote control of cellular behaviour with magnetic nanoparticles. Nat. Nanotechnol. 3, 139–143 (2008).
Huang, H., Delikanli, S., Zeng, H., Ferkey, D. M. & Pralle, A. Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol. 5, 602–606 (2010).
Legon, W. et al. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 17, 322–329 (2014).
Marino, A. et al. Piezoelectric nanoparticle-assisted wireless neuronal stimulation. ACS Nano 9, 7678–7689 (2015).
Tufail, Y. et al. Transcranial pulsed ultrasound stimulates intact brain circuits. Neuron 66, 681–694 (2010).
Tufail, Y., Yoshihiro, A., Pati, S., Li, M. M. & Tyler, W. J. Ultrasonic neuromodulation by brain stimulation with transcranial ultrasound. Nat. Protoc. 6, 1453–1470 (2011).
Tyler, W. J., Lani, S. W. & Hwang, G. M. Ultrasonic modulation of neural circuit activity. Curr. Opin. Neurobiol. 50, 222–231 (2018).
Tyler, W. J. et al. Remote excitation of neuronal circuits using low-intensity, low-frequency ultrasound. PLoS ONE 3, e3511 (2008).
Montgomery, K. L., Iyer, S. M., Christensen, A. J., Deisseroth, K. & Delp, S. L. Beyond the brain: optogenetic control in the spinal cord and peripheral nervous system. Sci. Transl. Med. 8, 337rv5 (2016).
Maimon, B. E., Sparks, K., Srinivasan, S., Zorzos, A. N. & Herr, H. M. Spectrally distinct channelrhodopsins for two-colour optogenetic peripheral nerve stimulation. Nat. Biomed. Eng. 2, 485–496 (2018).
Klapoetke, N. C. et al. Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346 (2014).
Lorach, H. et al. Photovoltaic restoration of sight with high visual acuity. Nat. Med. 21, 476–482 (2015).
Mandel, Y. et al. Cortical responses elicited by photovoltaic subretinal prostheses exhibit similarities to visually evoked potentials. Nat. Commun. 4, 1980 (2013).
Mathieson, K. et al. Photovoltaic retinal prosthesis with high pixel density. Nat. Photonics 6, 391–397 (2012).
Lugo, K., Miao, X., Rieke, F. & Lin, L. Y. Remote switching of cellular activity and cell signaling using light in conjunction with quantum dots. Biomed. Opt. Express 3, 447–454 (2012).
Pappas, T. C. et al. Nanoscale engineering of a cellular interface with semiconductor nanoparticle films for photoelectric stimulation of neurons. Nano Lett. 7, 513–519 (2007).
Rand, D. et al. Direct electrical neurostimulation with organic pigment photocapacitors. Adv. Mater. 30, e1707292 (2018).
Sytnyk, M. et al. Cellular interfaces with hydrogen-bonded organic semiconductor hierarchical nanocrystals. Nat. Commun. 8, 91 (2017).
Sato, T., Shapiro, M. G. & Tsao, D. Y. Ultrasonic neuromodulation causes widespread cortical activation via an indirect auditory mechanism. Neuron 98, 1031–1041 (2018).
Guo, H. et al. Ultrasound produces extensive brain activation via a cochlear pathway. Neuron 98, 1020–1030 (2018).
Yoo, S., Hong, S., Choi, Y., Park, J. H. & Nam, Y. Photothermal inhibition of neural activity with near-infrared-sensitive nanotransducers. ACS Nano 8, 8040–8049 (2014).
Rogers, J. A., Lagally, M. G. & Nuzzo, R. G. Synthesis, assembly and applications of semiconductor nanomembranes. Nature 477, 45–53 (2011).
Patolsky, F., Zheng, G. & Lieber, C. M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 1, 1711–1724 (2006).
Zhao, D. et al. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 279, 548–552 (1998).
Kleitz, F., Choi, S. H. & Ryoo, R. Cubic Ia3d large mesoporous silica: synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 2136-2137 (2003).
Fang, Y. et al. Texturing silicon nanowires for highly localized optical modulation of cellular dynamics. Nano Lett. 18, 4487–4492 (2018).
Merrill, D. R., Bikson, M. & Jefferys, J. G. Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J. Neurosci. Methods 141, 171–198 (2005).
Luo, Z. et al. Atomic gold-enabled three-dimensional lithography for silicon mesostructures. Science 348, 1451–1455 (2015).
Melli, G. & Hoke, A. Dorsal root ganglia sensory neuronal cultures: a tool for drug discovery for peripheral neuropathies. Expert Opin. Drug. Discov. 4, 1035–1045 (2009).
Oesterle, A. with Sutter Instrument Company. Pipette cookbook 2018: P-97 & P-1000 micropipette pullers. https://www.sutter.com/PDFs/pipette_cookbook.pdf (2018).
Molecular Devices. The Axon™ guide: a guide to electrophysiology and biophysics laboratory techniques. https://mdc.custhelp.com/euf/assets/content/Axon%20Guide%203rd%20edition.pdf (2012).
Molleman, A. Patch Clamping: An Introductory Guide to Patch Clamp Electrophysiology (John Wiley & Sons, Chichester, UK, 2003).
Rueda, A. G. Whole cell patch clamp recordings for characterizing neuronal electrical properties of iPSC-derive neurons. https://docs.axolbio.com/wp-content/uploads/patch-clamp-protocol-final.pdf (2018).
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 391, 85–100 (1981).
Edwards, F. A., Konnerth, A., Sakmann, B. & Takahashi, T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 414, 600–612 (1989).
Stuart, G. J., Dodt, H. U. & Sakmann, B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch. 423, 511–518 (1993).
Suter, B. A. et al. Ephus: multipurpose data acquisition software for neuroscience experiments. Front. Neural Circuits 4, 100 (2010).
Mostany, R. & Portera-Cailliau, C. A craniotomy surgery procedure for chronic brain imaging. J. Vis. Exp. 2008, e680 (2008).
Tang, J. et al. Nanowire arrays restore vision in blind mice. Nat. Commun. 9, 786 (2018).
Savchenko, A. et al. Graphene biointerfaces for optical stimulation of cells. Sci. Adv. 4, eaat0351 (2018).
Yao, J., Liu, B. & Qin, F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys. J. 96, 3611–3619 (2009).
Acknowledgements
This work was supported by the Air Force Office of Scientific Research (AFOSR FA9550-18-1-0503), the US Army Research Office (W911NF-18-1-0042), the US Office of Naval Research (N000141612530, N000141612958), the National Science Foundation (NSF MRSEC, DMR 1420709), the Searle Scholars Foundation, the National Institutes of Health (NIH NS101488, NS061963, GM030376, R21-EY023430, R21-EY027101), an MSTP Training Grant (T32GM007281), and the Paul and Daisy Soros Foundation. Atom-probe tomography was performed at the Northwestern University Center for Atom-Probe Tomography (NUCAPT), whose atom-probe tomography equipment was purchased and upgraded with funding from NSF-MRI (DMR-0420532) and ONR-DURIP (N00014-0400798, N00014-0610539, N00014-0910781) grants. NUCAPT is a Research Facility at the Materials Research Center of Northwestern University, supported by the National Science Foundation’s MRSEC program (grant DMR-1121262). Instrumentation at NUCAPT was further upgraded by the Initiative for Sustainability and Energy at Northwestern (ISEN). This work made use of the Japan Electron Optics Laboratory (JEOL) JEM-ARM200CF and JEOL JEM-3010 TEM in the Electron Microscopy Service of the Research Resources Center at the University of Illinois at Chicago (UIC). The acquisition of the UIC JEOL JEM-ARM200CF was supported by an MRI-R2 grant from the National Science Foundation (DMR-0959470).
Author information
Authors and Affiliations
Contributions
Y.J., R.P., X.L., J.L.C.-d.-S., F.B., G.M.G.S., and B.T. developed the protocol. Y.J., R.P., X.L., and J.L.C.-d.-S. performed the experiments. Y.J., R.P., X.L., and B.T. wrote the manuscript with input from J.L.C.-d.-S., X.G., L.M., F.B., and G.M.G.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Journal peer review information: Nature Protocols thanks Tal Dvir and other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Key references using this protocol
Jiang, Y. et al. Nat. Mater. 15, 1023–1030 (2016): https://doi.org/10.1038/nmat4673
Parameswaran, R. et al. Nat. Nanotechnol. 13, 260–266 (2018): https://doi.org/10.1038/s41565-017-0041-7
Jiang, Y. et al. Nat. Biomed. Eng. 2, 508–521 (2018): https://doi.org/10.1038/s41551-018-0230-1
Supplementary information
Supplementary Data 1
Mask design for the Si mesh structure
Supplementary Data 2
Mask design for the SU-8 pillar structure
Rights and permissions
About this article
Cite this article
Jiang, Y., Parameswaran, R., Li, X. et al. Nongenetic optical neuromodulation with silicon-based materials. Nat Protoc 14, 1339–1376 (2019). https://doi.org/10.1038/s41596-019-0135-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41596-019-0135-9
This article is cited by
-
Janus microparticles-based targeted and spatially-controlled piezoelectric neural stimulation via low-intensity focused ultrasound
Nature Communications (2024)
-
Non-Faradaic optoelectrodes for safe electrical neuromodulation
Nature Communications (2024)
-
Bioinspired nanotransducers for neuromodulation
Nano Research (2024)
-
Neural modulation with photothermally active nanomaterials
Nature Reviews Bioengineering (2023)
-
Porosity-based heterojunctions enable leadless optoelectronic modulation of tissues
Nature Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.