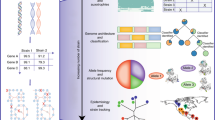
Abstract
Escherichia coli is considered to be the best-known microorganism given the large number of published studies detailing its genes, its genome and the biochemical functions of its molecular components. This vast literature has been systematically assembled into a reconstruction of the biochemical reaction networks that underlie E. coli’s functions, a process which is now being applied to an increasing number of microorganisms. Genome-scale reconstructed networks are organized and systematized knowledge bases that have multiple uses, including conversion into computational models that interpret and predict phenotypic states and the consequences of environmental and genetic perturbations. These genome-scale models (GEMs) now enable us to develop pan-genome analyses that provide mechanistic insights, detail the selection pressures on proteome allocation and address stress phenotypes. In this Review, we first discuss the overall development of GEMs and their applications. Next, we review the evolution of the most complete GEM that has been developed to date: the E. coli GEM. Finally, we explore three emerging areas in genome-scale modelling of microbial phenotypes: collections of strain-specific models, metabolic and macromolecular expression models, and simulation of stress responses.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Thiele, I. & Palsson, B. Ø. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat. Protoc. 5, 93–121 (2010). A detailed protocol to reconstruct a GEM from a genome sequence.
Norsigian, C. J., Fang, X., Seif, Y., Monk, J. M. & Palsson, B. O. A workflow for generating multi-strain genome-scale metabolic models of prokaryotes. Nat. Protoc. 15, 1–14 (2020). This article provides a semi-automated workflow to generate strain-specific GEMs from a curated reference model.
Price, N. D., Reed, J. L. & Palsson, B. Ø. Genome-scale models of microbial cells: evaluating the consequences of constraints. Nat. Rev. Microbiol. 2, 886–897 (2004).
Lewis, N. E., Nagarajan, H. & Palsson, B. O. Constraining the metabolic genotype–phenotype relationship using a phylogeny of in silico methods. Nat. Rev. Microbiol. 10, 291–305 (2012).
Kanehisa, M. & Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Feist, A. M. & Palsson, B. O. The biomass objective function. Curr. Opin. Microbiol. 13, 344–349 (2010).
Orth, J. D., Thiele, I. & Palsson, B. Ø. What is flux balance analysis? Nat. Biotechnol. 28, 245 (2010). This is primer that thoroughly explains FBA and its applications.
Ebrahim, A., Lerman, J. A., Palsson, B. O. & Hyduke, D. R. COBRApy: constraints-based reconstruction and analysis for python. BMC Syst. Biol. 7, 74 (2013).
Heirendt, L. et al. Creation and analysis of biochemical constraint-based models using the COBRA Toolbox v.3.0. Nat. Protoc. 14, 639–702 (2019).
Feist, A. M. & Palsson, B. Ø. The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli. Nat. Biotechnol. 26, 659–667 (2008).
McCloskey, D., Palsson, B. Ø. & Feist, A. M. Basic and applied uses of genome-scale metabolic network reconstructions of Escherichia coli. Mol. Syst. Biol. 9, 661 (2013).
Bordbar, A., Monk, J. M., King, Z. A. & Palsson, B. O. Constraint-based models predict metabolic and associated cellular functions. Nat. Rev. Genet. 15, 107–120 (2014).
O’Brien, E. J., Monk, J. M. & Palsson, B. O. Using genome-scale models to predict biological capabilities. Cell 161, 971–987 (2015).
Gu, C., Kim, G. B., Kim, W. J., Kim, H. U. & Lee, S. Y. Current status and applications of genome-scale metabolic models. Genome Biol. 20, 121 (2019).
Oberhardt, M. A., Palsson, B. Ø. & Papin, J. A. Applications of genome-scale metabolic reconstructions. Mol. Syst. Biol. 5, 320 (2009).
Mazumdar, V., Snitkin, E. S., Amar, S. & Segrè, D. Metabolic network model of a human oral pathogen. J. Bacteriol. 191, 74–90 (2009).
Henry, C. S., Jankowski, M. D., Broadbelt, L. J. & Hatzimanikatis, V. Genome-scale thermodynamic analysis of Escherichia coli metabolism. Biophys. J. 90, 1453–1461 (2006).
Navid, A. & Almaas, E. Genome-level transcription data of Yersinia pestis analyzed with a new metabolic constraint-based approach. BMC Syst. Biol. 6, 150 (2012).
Motter, A. E., Gulbahce, N., Almaas, E. & Barabási, A.-L. Predicting synthetic rescues in metabolic networks. Mol. Syst. Biol. 4, 168 (2008).
Montagud, A., Navarro, E., Fernández de Córdoba, P., Urchueguía, J. F. & Patil, K. R. Reconstruction and analysis of genome-scale metabolic model of a photosynthetic bacterium. BMC Syst. Biol. 4, 156 (2010).
Mahadevan, R., Edwards, J. S. & Doyle, F. J. III. Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J. 83, 1331–1340 (2002).
Hastings, J. et al. Multi-omics and genome-scale modeling reveal a metabolic shift during C. elegans aging. Front. Mol. Biosci. 6, 2 (2019).
Guan, N. et al. Comparative genomics and transcriptomics analysis-guided metabolic engineering of Propionibacterium acidipropionici for improved propionic acid production. Biotechnol. Bioeng. 115, 483–494 (2018).
Burgard, A. P., Pharkya, P. & Maranas, C. D. Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol. Bioeng. 84, 647–657 (2003).
Hefzi, H. et al. A consensus genome-scale reconstruction of Chinese hamster ovary cell metabolism. Cell Syst. 3, 434–443.e8 (2016).
Cautha, S. C. et al. Model-driven design of a Saccharomyces cerevisiae platform strain with improved tyrosine production capabilities. IFAC Proc. Vol. 46, 221–226 (2013).
McAnulty, M. J., Yen, J. Y., Freedman, B. G. & Senger, R. S. Genome-scale modeling using flux ratio constraints to enable metabolic engineering of clostridial metabolism in silico. BMC Syst. Biol. 6, 42 (2012).
Cardoso, J. G. R., Andersen, M. R., Herrgård, M. J. & Sonnenschein, N. Analysis of genetic variation and potential applications in genome-scale metabolic modeling. Front. Bioeng. Biotechnol. 3, 13 (2015).
Folger, O. et al. Predicting selective drug targets in cancer through metabolic networks. Mol. Syst. Biol. 7, 501 (2011).
Guzmán, G. I. et al. Model-driven discovery of underground metabolic functions in Escherichia coli. Proc. Natl Acad. Sci. USA 112, 929–934 (2015).
Kumar, M., Ji, B., Zengler, K. & Nielsen, J. Modelling approaches for studying the microbiome. Nat. Microbiol. 4, 1253–1267 (2019).
Zuñiga, C. et al. Environmental stimuli drive a transition from cooperation to competition in synthetic phototrophic communities. Nat. Microbiol. 4, 2184–2191 (2019).
Zomorrodi, A. R. & Maranas, C. D. OptCom: a multi-level optimization framework for the metabolic modeling and analysis of microbial communities. PLoS Comput. Biol. 8, e1002363 (2012).
Stolyar, S. et al. Metabolic modeling of a mutualistic microbial community. Mol. Syst. Biol. 3, 92 (2007).
Zhuang, K. et al. Genome-scale dynamic modeling of the competition between Rhodoferax and Geobacter in anoxic subsurface environments. ISME J. 5, 305–316 (2011).
Harcombe, W. R. et al. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 7, 1104–1115 (2014).
Zomorrodi, A. R. & Segrè, D. Genome-driven evolutionary game theory helps understand the rise of metabolic interdependencies in microbial communities. Nat. Commun. 8, 1563 (2017).
Zelezniak, A. et al. Metabolic dependencies drive species co-occurrence in diverse microbial communities. Proc. Natl Acad. Sci. USA 112, 6449–6454 (2015).
Chowdhury, S. & Fong, S. S. Computational modeling of the human microbiome. Microorganisms 8, 197 (2020).
Levy, R. & Borenstein, E. Metabolic modeling of species interaction in the human microbiome elucidates community-level assembly rules. Proc. Natl Acad. Sci. USA 110, 12804–12809 (2013).
Mardinoglu, A. et al. Genome-scale metabolic modelling of hepatocytes reveals serine deficiency in patients with non-alcoholic fatty liver disease. Nat. Commun. 5, 3083 (2014).
Mardinoglu, A. & Nielsen, J. Systems medicine and metabolic modelling. J. Intern. Med. 271, 142–154 (2012).
Edwards, J. S. & Palsson, B. O. Systems properties of the Haemophilus influenzae Rd metabolic genotype. J. Biol. Chem. 274, 17410–17416 (1999).
Monk, J., Nogales, J. & Palsson, B. O. Optimizing genome-scale network reconstructions. Nat. Biotechnol. 32, 447–452 (2014).
Mendoza, S. N., Olivier, B. G., Molenaar, D. & Teusink, B. A systematic assessment of current genome-scale metabolic reconstruction tools. Genome Biol. 20, 158 (2019).
Satish Kumar, V., Ferry, J. G. & Maranas, C. D. Metabolic reconstruction of the archaeon methanogen Methanosarcina acetivorans. BMC Syst. Biol. 5, 28 (2011).
Benedict, M. N., Gonnerman, M. C., Metcalf, W. W. & Price, N. D. Genome-scale metabolic reconstruction and hypothesis testing in the methanogenic archaeon Methanosarcina acetivorans C2A. J. Bacteriol. 194, 855–865 (2012).
Peterson, J. R. et al. Genome-wide gene expression and RNA half-life measurements allow predictions of regulation and metabolic behavior in Methanosarcina acetivorans. BMC Genomics 17, 924 (2016).
Sheikh, K., Förster, J. & Nielsen, L. K. Modeling hybridoma cell metabolism using a generic genome-scale metabolic model of Mus musculus. Biotechnol. Prog. 21, 112–121 (2005).
Lu, H. et al. A consensus S. cerevisiae metabolic model Yeast8 and its ecosystem for comprehensively probing cellular metabolism. Nat. Commun. 10, 3586 (2019).
de Oliveira Dal’Molin, C. G. & Nielsen, L. K. Plant genome-scale metabolic reconstruction and modelling. Curr. Opin. Biotechnol. 24, 271–277 (2013).
Mintz-Oron, S. et al. Reconstruction of Arabidopsis metabolic network models accounting for subcellular compartmentalization and tissue-specificity. Proc. Natl Acad. Sci. USA 109, 339–344 (2012).
Cheung, C. Y. M. et al. A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant. J. 75, 1050–1061 (2013).
Thiele, I. et al. A community-driven global reconstruction of human metabolism. Nat. Biotechnol. 31, 419–425 (2013).
Swainston, N. et al. Recon 2.2: from reconstruction to model of human metabolism. Metabolomics 12, 109 (2016).
Brunk, E. et al. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat. Biotechnol. 36, 272–281 (2018).
Kumar, A., Suthers, P. F. & Maranas, C. D. MetRxn: a knowledgebase of metabolites and reactions spanning metabolic models and databases. BMC Bioinformatics 13, 6 (2012).
Henry, C. S. et al. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat. Biotechnol. 28, 977–982 (2010).
Machado, D., Andrejev, S., Tramontano, M. & Patil, K. R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 46, 7542–7553 (2018).
Agren, R. et al. The RAVEN toolbox and its use for generating a genome-scale metabolic model for Penicillium chrysogenum. PLoS Comput. Biol. 9, e1002980 (2013).
Wang, H. et al. RAVEN 2.0: a versatile toolbox for metabolic network reconstruction and a case study on Streptomyces coelicolor. PLoS Comput. Biol. 14, e1006541 (2018).
Arkin, A. P. et al. KBase: the United States department of energy systems biology knowledgebase. Nat. Biotechnol. 36, 566–569 (2018).
Norsigian, C. J. et al. BiGG Models 2020: multi-strain genome-scale models and expansion across the phylogenetic tree. Nucleic Acids Res. 48, D402–D406 (2019).
Glont, M. et al. BioModels: expanding horizons to include more modelling approaches and formats. Nucleic Acids Res. 46, D1248–D1253 (2018).
Moretti, S. et al. MetaNetX/MNXref–reconciliation of metabolites and biochemical reactions to bring together genome-scale metabolic networks. Nucleic Acids Res. 44, D523–D526 (2016).
Pabinger, S. et al. MEMOSys 2.0: an update of the bioinformatics database for genome-scale models and genomic data. Database 2014, bau004 (2014).
Noronha, A. et al. The virtual metabolic human database: integrating human and gut microbiome metabolism with nutrition and disease. Nucleic Acids Res. 47, D614–D624 (2019).
Lieven, C. et al. MEMOTE for standardized genome-scale metabolic model testing. Nat. Biotechnol. 38, 272–276 (2020).
Varma, A. & Palsson, B. O. Metabolic capabilities of Escherichia coli II. Optimal growth patterns. J. Theor. Biol. 165, 503–522 (1993).
Pramanik, J. & Keasling, J. D. Stoichiometric model of Escherichia coli metabolism: incorporation of growth-rate dependent biomass composition and mechanistic energy requirements. Biotechnol. Bioeng. 56, 398–421 (1997).
Pramanik, J. & Keasling, J. D. Effect of Escherichia coli biomass composition on central metabolic fluxes predicted by a stoichiometric model. Biotechnol. Bioeng. 60, 230–238 (1998).
Edwards, J. S. & Palsson, B. O. The Escherichia coli MG1655 in silico metabolic genotype: its definition, characteristics, and capabilities. Proc. Natl Acad. Sci. USA 97, 5528–5533 (2000).
Reed, J. L., Vo, T. D., Schilling, C. H. & Palsson, B. O. An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR). Genome Biol. 4, R54 (2003).
Feist, A. M. et al. A genome-scale metabolic reconstruction for Escherichia coli K-12 MG1655 that accounts for 1260 ORFs and thermodynamic information. Mol. Syst. Biol. 3, 121 (2007).
Orth, J. D. et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism — 2011. Mol. Syst. Biol. 7, 535 (2011).
Monk, J. M. et al. iML1515, a knowledgebase that computes Escherichia coli traits. Nat. Biotechnol. 35, 904–908 (2017). This article provides the most up-to-date genome-scale metabolic model of E. coli.
Archer, C. T. et al. The genome sequence of E. coli W (ATCC 9637): comparative genome analysis and an improved genome-scale reconstruction of E. coli. BMC Genomics 12, 9 (2011).
Blattner, F. R. et al. The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 (1997).
Brunk, E. et al. Systems biology of the structural proteome. BMC Syst. Biol. 10, 26 (2016).
Mih, N. et al. ssbio: a Python framework for structural systems biology. Bioinformatics 34, 2155–2157 (2018).
Ghatak, S., King, Z. A., Sastry, A. & Palsson, B. O. The y-ome defines the 35% of Escherichia coli genes that lack experimental evidence of function. Nucleic Acids Res. 47, 2446–2454 (2019).
Mih, N. & Palsson, B. O. Expanding the uses of genome-scale models with protein structures. Mol. Syst. Biol. 15, e8601 (2019).
Magnúsdóttir, S. & Thiele, I. Modeling metabolism of the human gut microbiome. Curr. Opin. Biotechnol. 51, 90–96 (2018).
Opdam, S. et al. A systematic evaluation of methods for tailoring genome-scale metabolic models. Cell Syst. 4, 318–329.e6 (2017).
Rouli, L., Merhej, V., Fournier, P.-E. & Raoult, D. The bacterial pangenome as a new tool for analysing pathogenic bacteria. New Microbes New Infect. 7, 72–85 (2015).
Lukjancenko, O., Wassenaar, T. M. & Ussery, D. W. Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 60, 708–720 (2010).
Seif, Y. et al. Genome-scale metabolic reconstructions of multiple Salmonella strains reveal serovar-specific metabolic traits. Nat. Commun. 9, 3771 (2018).
John, J., George, S., Nori, S. R. C. & Nelson-Sathi, S. Phylogenomic analysis reveals the evolutionary route of resistant genes in Staphylococcus aureus. Genome Biol. Evol. 11, 2917–2926 (2019).
Holt, K. E. et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl Acad. Sci. USA 112, E3574–E3581 (2015).
Yang, Z.-K., Luo, H., Zhang, Y., Wang, B. & Gao, F. Pan-genomic analysis provides novel insights into the association of E.coli with human host and its minimal genome. Bioinformatics 35, 1987–1991 (2019).
Monk, J. M. et al. Genome-scale metabolic reconstructions of multiple Escherichia coli strains highlight strain-specific adaptations to nutritional environments. Proc. Natl Acad. Sci. USA 110, 20338–20343 (2013). This is the first study to focus on a strain-level study of E. coli using GEMs.
Fang, X. et al. Escherichia coli B2 strains prevalent in inflammatory bowel disease patients have distinct metabolic capabilities that enable colonization of intefstinal mucosa. BMC Syst. Biol. 12, 66 (2018).
Fang, X. et al. Metagenomics-based, strain-level analysis of Escherichia coli from a time-series of microbiome samples from a Crohn’s disease patient. Front. Microbiol. 9, 2559 (2018).
Bosi, E. et al. Comparative genome-scale modelling of Staphylococcus aureus strains identifies strain-specific metabolic capabilities linked to pathogenicity. Proc. Natl Acad. Sci. USA 113, E3801–E3809 (2016).
Seiler, N. & Atanassov, C. L. The natural polyamines and the immune system. Prog. Drug Res. 43, 87–141 (1994).
Norsigian, C. J. et al. Comparative genome-scale metabolic modeling of metallo-beta-lactamase–producing multidrug-resistant Klebsiella pneumoniae clinical isolates. Front. Cell. Infect. Microbiol. 9, 161 (2019).
Norsigian, C. J., Kavvas, E., Seif, Y., Palsson, B. O. & Monk, J. M. iCN718, an updated and improved genome-scale metabolic network reconstruction of Acinetobacter baumannii AYE. Front. Genet. 9, 121 (2018).
Fouts, D. E. et al. What makes a bacterial species pathogenic?: Comparative genomic analysis of the genus Leptospira. PLoS Negl. Trop. Dis. 10, e0004403 (2016).
Nogales, J. et al. High-quality genome-scale metabolic modelling of Pseudomonas putida highlights its broad metabolic capabilities. Environ. Microbiol. 22, 255–269 (2020).
Kavvas, E. S., Yang, L., Monk, J. M., Heckmann, D. & Palsson, B. O. A biochemically-interpretable machine learning classifier for microbial GWAS. Nat. Commun. 11, 2580 (2020).
Sánchez, B. J. et al. Improving the phenotype predictions of a yeast genome-scale metabolic model by incorporating enzymatic constraints. Mol. Syst. Biol. 13, 935 (2017).
Carrera, J. et al. An integrative, multi-scale, genome-wide model reveals the phenotypic landscape of Escherichia coli. Mol. Syst. Biol. 10, 735 (2014).
Thiele, I., Jamshidi, N., Fleming, R. M. T. & Palsson, B. Ø. Genome-scale reconstruction of Escherichia coli’s transcriptional and translational machinery: a knowledge base, its mathematical formulation, and its functional characterization. PLoS Comput. Biol. 5, e1000312 (2009).
Milo, R. & Phillips, R. Cell Biology by the Numbers (Garland Science, 2015).
Lerman, J. A. et al. In silico method for modelling metabolism and gene product expression at genome scale. Nat. Commun. 3, 929 (2012).
Liu, J. K., O’Brien, E. J. & Lerman, J. A. Reconstruction and modeling protein translocation and compartmentalization in Escherichia coli at the genome-scale. BMC Syst. Biol. 8, 110 (2014).
Heckmann, D. et al. Machine learning applied to enzyme turnover numbers reveals protein structural correlates and improves metabolic models. Nat. Commun. 9, 5252 (2018).
Lloyd, C. J. et al. COBRAme: a computational framework for genome-scale models of metabolism and gene expression. PLoS Comput. Biol. 14, e1006302 (2018). This article provides a framework that streamlines ME model reconstruction and analysis.
Liu, J. K. et al. Predicting proteome allocation, overflow metabolism, and metal requirements in a model acetogen. PLoS Comput. Biol. 15, e1006848 (2019).
Salvy, P. & Hatzimanikatis, V. The ETFL formulation allows multi-omics integration in thermodynamics-compliant metabolism and expression models. Nat. Commun. 11, 30 (2020). This article describes an implementation of the ME models called ‘ETFL’, for ‘expression and thermodynamics flux models’.
Salvy, P. & Hatzimanikatis, V. Emergence of diauxie as an optimal growth strategy under resource allocation constraints in cellular metabolism. Preprint at bioRxiv https://doi.org/10.1101/2020.07.15.204420 (2020).
Caspi, R. et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of pathway/genome databases. Nucleic Acids Res. 44, D471–D480 (2016).
Nilsson, A., Nielsen, J. & Palsson, B. O. Metabolic models of protein allocation call for the kinetome. Cell Syst. 5, 538–541 (2017).
Heckmann, D. et al. Kinetic profiling of metabolic specialists demonstrates stability and consistency of in vivo enzyme turnover numbers. Proc. Natl Acad. Sci. USA 117, 23182–23190 (2020).
Karr, J. R. et al. A whole-cell computational model predicts phenotype from genotype. Cell 150, 389–401 (2012).
Ye, C. et al. Comprehensive understanding of Saccharomyces cerevisiae phenotypes with whole-cell model WM_S288C. Biotechnol. Bioeng. 117, 1562–1574 (2020).
Macklin, D. N. et al. Simultaneous cross-evaluation of heterogeneous E. coli datasets via mechanistic simulation. Science 369, eaav3751 (2020).
Xu, P., Ranganathan, S., Fowler, Z. L., Maranas, C. D. & Koffas, M. A. G. Genome-scale metabolic network modeling results in minimal interventions that cooperatively force carbon flux towards malonyl-CoA. Metab. Eng. 13, 578–587 (2011).
Akesson, M., Förster, J. & Nielsen, J. Integration of gene expression data into genome-scale metabolic models. Metab. Eng. 6, 285–293 (2004).
Yim, H. et al. Metabolic engineering of Escherichia coli for direct production of 1,4-butanediol. Nat. Chem. Biol. 7, 445–452 (2011).
Wang, Q., Chen, X., Yang, Y. & Zhao, X. Genome-scale in silico aided metabolic analysis and flux comparisons of Escherichia coli to improve succinate production. Appl. Microbiol. Biotechnol. 73, 887–894 (2006).
Imielinski, M., Belta, C., Rubin, H. & Halász, A. Systematic analysis of conservation relations in Escherichia coli genome-scale metabolic network reveals novel growth media. Biophys. J. 90, 2659–2672 (2006).
Yang, L. et al. Systems biology definition of the core proteome of metabolism and expression is consistent with high-throughput data. Proc. Natl Acad. Sci. USA 112, 10810–10815 (2015).
O’brien, E. J., Lerman, J. A., Chang, R. L., Hyduke, D. R. & Palsson, B. Ø. Genome-scale models of metabolism and gene expression extend and refine growth phenotype prediction. Mol. Syst. Biol. 9, 693 (2013). This study outlines the development of the first E. coli ME model.
O’Brien, E. J., Utrilla, J. & Palsson, B. O. Quantification and classification of E. coli proteome utilization and unused protein costs across environments. PLoS Comput. Biol. 12, e1004998 (2016).
Lloyd, C. J., Monk, J., Yang, L., Ebrahim, A. & Palsson, B. O. Computation of condition-dependent proteome allocation reveals variability in the macro and micro nutrient requirements for growth. Preprint at bioRxiv https://doi.org/10.1101/2020.03.23.003236 (2020).
Du, B., Yang, L., Lloyd, C. J., Fang, X. & Palsson, B. O. Genome-scale model of metabolism and gene expression provides a multi-scale description of acid stress responses in Escherichia coli. PLoS Comput. Biol. 15, e1007525 (2019). As one of the three stress response extensions of ME model, this study focuses on modelling response to acid stress.
Chen, K. et al. Thermosensitivity of growth is determined by chaperone-mediated proteome reallocation. Proc. Natl Acad. Sci. USA 114, 11548–11553 (2017). As one of the three stress response extensions of the ME model, FoldME focuses on simulation of response to temperature stress.
Yang, L. et al. Cellular responses to reactive oxygen species are predicted from molecular mechanisms. Proc. Natl Acad. Sci. USA 116, 14368–14373 (2019). As one of the three stress response extensions of the ME model, OxidizeME incorporates mechanisms involved in response to oxidative stress.
Mayr, E. This is Biology: The Science of the Living World (Harvard Univ. Press, 1998).
Palsson, B. Systems Biology (Cambridge Univ. Press, 2015).
Davis, J. J. et al. The PATRIC bioinformatics resource center: expanding data and analysis capabilities. Nucleic Acids Res. 48, D606–D612 (2020).
Bailey, J. E. Mathematical modeling and analysis in biochemical engineering: past accomplishments and future opportunities. Biotechnol. Prog. 14, 8–20 (1998).
Covert, M. W., Knight, E. M., Reed, J. L., Herrgard, M. J. & Palsson, B. O. Integrating high-throughput and computational data elucidates bacterial networks. Nature 429, 92 (2004).
Mahadevan, R., Palsson, B. Ø. & Lovley, D. R. In situ to in silico and back: elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nat. Rev. Microbiol. 9, 39–50 (2011).
Kim, H. U. et al. Integrative genome-scale metabolic analysis of Vibrio vulnificus for drug targeting and discovery. Mol. Syst. Biol. 7, 460 (2011).
Luo, H. et al. Coupling S-adenosylmethionine-dependent methylation to growth: design and uses. PLoS Biol. 17, e2007050 (2019).
Reed, J. L. et al. Systems approach to refining genome annotation. Proc. Natl Acad. Sci. USA 103, 17480–17484 (2006).
Kumar, V. S. & Maranas, C. D. GrowMatch: an automated method for reconciling in silico/in vivo growth predictions. PLoS Comput. Biol. 5, e1000308 (2009).
Orth, J. D. & Palsson, B. Ø. Systematizing the generation of missing metabolic knowledge. Biotechnol. Bioeng. 107, 403–412 (2010).
Medlock, G. L. & Papin, J. A. Guiding the refinement of biochemical knowledgebases with ensembles of metabolic networks and machine learning. Cell Syst. 10, 109–119.e3 (2020).
Acknowledgements
This work was supported by the US National Institutes of General Medical Sciences of the National Institutes of Health (grant R01GM057089) and the Novo Nordisk Foundation (grant NNF10CC1016517). Some figures were adapted from previously published figures, and the authors acknowledge the original authors of these figures and thank them for their contributions: J. M. Monk (Fig. 3), L. Yang (Fig. 5), K. Chen (Fig. 5), B. Du (Fig. 5) and A. Bordbar (Fig. 1).
Author information
Authors and Affiliations
Contributions
X.F. researched data for the article. X.F. and B.O.P. substantially contributed to discussion of the content. All authors wrote the article, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks María Suárez Diez, Sara Moreno Paz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Python
-
An interpreted, general-purpose programming language that is widely used in computational biology.
- Reactomes
-
All the reactions involved in genome-scale models (or a certain model of interest). Each base unit is a reaction, and the entities are metabolites involved in the reactions, such as proteins, nucleic acids and small molecules.
- Proteome allocation
-
The partition of proteomics resources into different functions to fulfil the organism’s need under the given condition.
- Sensome
-
The components (such as genes and proteins) in an organism or cell that are involved in sensing the changes in the environment.
- Proximal causation
-
Explains traits/events (such as change in proteome allocation) in terms of immediate physiological or environmental factors.
- Distal causation
-
Explains traits/events (such as change in proteome allocation) in terms of evolutionary forces acting on them.
- Expression matrix
-
(E matrix). A matrix that describes all components (including DNA, mRNA, proteins and metabolites) and reactions that are involved in the transcriptional and translational machinery in the organism of interest.
- Overflow metabolism
-
When cells incompletely oxidize their substrate (which yields less energy) instead of using the more energetically efficient respiratory pathways to completely oxidize their substrates even when oxygen is available.
Rights and permissions
About this article
Cite this article
Fang, X., Lloyd, C.J. & Palsson, B.O. Reconstructing organisms in silico: genome-scale models and their emerging applications. Nat Rev Microbiol 18, 731–743 (2020). https://doi.org/10.1038/s41579-020-00440-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-020-00440-4
This article is cited by
-
Reconstruction, simulation and analysis of enzyme-constrained metabolic models using GECKO Toolbox 3.0
Nature Protocols (2024)
-
The TAM, a Translocation and Assembly Module for protein assembly and potential conduit for phospholipid transfer
EMBO Reports (2024)
-
Reconstruction of genome-scale metabolic models of non-conventional yeasts: current state, challenges, and perspectives
Biotechnology and Bioprocess Engineering (2024)
-
Epistasis and evolution: recent advances and an outlook for prediction
BMC Biology (2023)
-
Novel context-specific genome-scale modelling explores the potential of triacylglycerol production by Chlamydomonas reinhardtii
Microbial Cell Factories (2023)