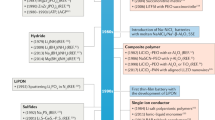
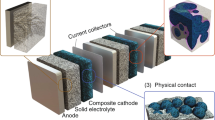
Abstract
Solid-state batteries (SSBs) using a solid electrolyte show potential for providing improved safety as well as higher energy and power density compared with conventional Li-ion batteries. However, two critical bottlenecks remain: the development of solid electrolytes with ionic conductivities comparable to or higher than those of conventional liquid electrolytes and the creation of stable interfaces between SSB components, including the active material, solid electrolyte and conductive additives. Although the first goal has been achieved in several solid ionic conductors, the high impedance at various solid/solid interfaces remains a challenge. Recently, computational models based on ab initio calculations have successfully predicted the stability of solid electrolytes in various systems. In addition, a large amount of experimental data has been accumulated for different interfaces in SSBs. In this Review, we summarize the experimental findings for various classes of solid electrolytes and relate them to computational predictions, with the aim of providing a deeper understanding of the interfacial reactions and insight for the future design and engineering of interfaces in SSBs. We find that, in general, the electrochemical stability and interfacial reaction products can be captured with a small set of chemical and physical principles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
09 March 2020
A Correction to this paper has been published: https://doi.org/10.1038/s41578-020-0191-3
References
Arora, P., White, R. E. & Doyle, M. Capacity fade mechanisms and side reactions in lithium-ion batteries. J. Electrochem. Soc. 145, 3647–3667 (1998).
Vetter, J. et al. Ageing mechanisms in lithium-ion batteries. J. Power Sources 147, 269–281 (2005).
Li, J., Ma, C., Chi, M., Liang, C. & Dudney, N. J. Solid electrolyte: the key for high-voltage lithium batteries. Adv. Energy Mater. 5, 1401408 (2015).
Lin, D., Liu, Y. & Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 12, 194–206 (2017).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 9, 504–510 (2010).
Epelboin, I., Froment, M., Garreau, M., Thevenin, J. & Warin, D. Behavior of secondary lithium and aluminum-lithium electrodes in propylene carbonate. J. Electrochem. Soc. 127, 2100–2104 (1980).
Han, F., Yue, J., Zhu, X. & Wang, C. Suppressing Li dendrite formation in Li2S–P2S5 solid electrolyte by LiI incorporation. Adv. Energy Mater. 8, 1703644 (2018).
Yu, X., Bates, J. B., Jellison, G. E. & Hart, F. X. A stable thin-film lithium electrolyte: lithium phosphorus oxynitride. J. Electrochem. Soc. 144, 524–532 (1997).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Ren, Y., Shen, Y., Lin, Y. & Nan, C.-W. Direct observation of lithium dendrites inside garnet-type lithium-ion solid electrolyte. Electrochem. Commun. 57, 27–30 (2015).
Ponrouch, A., Marchante, E., Courty, M., Tarascon, J.-M. & Rosa Palacín, M. In search of an optimized electrolyte for Na-ion batteries. Energy Environ. Sci. 5, 8572–8583 (2012).
Valøen, L. O. & Reimers, J. N. Transport properties of LiPF6-based Li-ion battery electrolytes. J. Electrochem. Soc. 152, A882–A891 (2005).
Aono, H., Sugimoto, E., Sadaoka, Y., Imanaka, N. & Adachi, G.-y Ionic conductivity of solid electrolytes based on lithium titanium phosphate. J. Electrochem. Soc. 137, 1023–1027 (1990).
Fergus, J. W. Ion transport in sodium ion conducting solid electrolytes. Solid State Ion. 227, 102–112 (2012).
Goodenough, J. B., Hong, H. Y.-P. & Kafalas, J. A. Fast Na+-ion transport in skeleton structures. Mater. Res. Bull. 11, 203–220 (1976).
Khireddine, H., Fabry, P., Caneiro, A. & Bochu, B. Optimization of NASICON composition for Na+ recognition. Sens. Actuators B Chem. 40, 223–230 (1997).
Subramanian, M., Subramanian, R. & Clearfield, A. Lithium ion conductors in the system AB(IV)2(PO4)3 (B = Ti, Zr and Hf). Solid State Ion. 18, 562–569 (1986).
Von Alpen, U., Bell, M. F. & Höfer, H. H. Compositional dependence of the electrochemical and structural parameters in the Nasicon system (Na1+xSixZr2P3−xO12). Solid State Ion. 3, 215–218 (1981).
Briant, J. L. & Farrington, G. C. Ionic conductivity in lithium and lithium sodium beta alumina. J. Electrochem. Soc. 128, 1830–1834 (1981).
Lu, X., Lemmon, J. P., Sprenkle, V. & Yang, Z. Sodium-beta alumina batteries: status and challenges. JOM 62, 31–36 (2010).
Whittingham, M. S. & Huggins, R. A. Measurement of sodium ion transport in beta alumina using reversible solid electrodes. J. Chem. Phys. 54, 414–416 (1971).
Yao, Y.-F. Y. & Kummer, J. T. Ion exchange properties of and rates of ionic diffusion in beta-alumina. J. Inorg. Nucl. Chem. 29, 2453–2475 (1967).
Allen, J. L., Wolfenstine, J., Rangasamy, E. & Sakamoto, J. Effect of substitution (Ta, Al, Ga) on the conductivity of Li7La3Zr2O12. J. Power Sources 206, 315–319 (2012).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007).
Ohta, S., Kobayashi, T. & Asaoka, T. High lithium ionic conductivity in the garnet-type oxide Li7−XLa3(Zr2−X, NbX)O12 (X=0–2). J. Power Sources 196, 3342–3345 (2011).
Thangadurai, V., Narayanan, S. & Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: critical review. Chem. Soc. Rev. 43, 4714–4727 (2014).
Stramare, S., Thangadurai, V. & Weppner, W. Lithium lanthanum titanates: a review. Chem. Mater. 15, 3974–3990 (2003).
Zhao, Y. & Daemen, L. L. Superionic conductivity in lithium-rich anti-perovskites. J. Am. Chem. Soc. 134, 15042–15047 (2012).
Kanno, R. & Murayama, M. Lithium ionic conductor thio-LISICON: the Li2S–GeS2–P2S5 system. J. Electrochem. Soc. 148, A742–A746 (2001).
Murayama, M., Sonoyama, N., Yamada, A. & Kanno, R. Material design of new lithium ionic conductor, thio-LISICON, in the Li2S–P2S5 system. Solid State Ion. 170, 173–180 (2004).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Kato, Y. et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 1, 16030 (2016).
Ong, S. P. et al. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M = Ge, Si, Sn, Al or P, and X = O, S or Se) family of superionic conductors. Energy Environ. Sci. 6, 148–156 (2013).
Hayashi, A., Hama, S., Morimoto, H., Tatsumisago, M. & Minami, T. Preparation of Li2S–P2S5 amorphous solid electrolytes by mechanical milling. J. Am. Ceram. Soc. 84, 477–479 (2001).
Seino, Y., Ota, T., Takada, K., Hayashi, A. & Tatsumisago, M. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries. Energy Environ. Sci. 7, 627–631 (2014).
Deiseroth, H.-J. et al. Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility. Angew. Chem. Int. Ed. 47, (755–758 (2008).
Rao, R. P. & Adams, S. Studies of lithium argyrodite solid electrolytes for all-solid-state batteries. Phys. Status Solidi A 208, 1804–1807 (2011).
Hayashi, A., Noi, K., Sakuda, A. & Tatsumisago, M. Superionic glass-ceramic electrolytes for room-temperature rechargeable sodium batteries. Nat. Commun. 3, 856 (2012).
Jansen, M. & Henseler, U. Synthesis, structure determination, and ionic conductivity of sodium tetrathiophosphate. J. Solid State Chem. 99, 110–119 (1992).
Bo, S. H., Wang, Y., Kim, J. C., Richards, W. D. & Ceder, G. Computational and experimental investigations of Na-ion conduction in cubic Na3PSe4. Chem. Mater. 28, 252–258 (2016).
Banerjee, A. et al. Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. Int. Ed. 128, 9786–9790 (2016).
Richards, W. D. et al. Design and synthesis of the superionic conductor Na10SnP2S12. Nat. Commun. 7, 11009 (2016).
Zhang, Z. et al. Na11Sn2PS12: a new solid state sodium superionic conductor. Energy Environ. Sci. 11, 87–93 (2018).
Tang, W. S. et al. Liquid-like ionic conduction in solid lithium and sodium monocarba-closo-decaborates near or at room temperature. Adv. Energy Mater. 6, 1502237 (2016).
Udovic, T. J. et al. Exceptional superionic conductivity in disordered sodium decahydro-closo-decaborate. Adv. Mater. 26, 7622–7626 (2014).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Interfacial observation between LiCoO2 electrode and Li2S–P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy. Chem. Mater. 22, 949–956 (2010).
Takada, K. Progress and prospective of solid-state lithium batteries. Acta Mater. 61, 759–770 (2013).
Takada, K. et al. Interfacial phenomena in solid-state lithium battery with sulfide solid electrolyte. Solid State Ion. 225, 594–597 (2012).
Tian, Y. et al. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci. 10, 1150–1166 (2017).
Wenzel, S. et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode. Chem. Mater. 28, 2400–2407 (2016).
Whiteley, J. M., Woo, J. H., Hu, E., Nam, K.-W. & Lee, S.-H. Empowering the lithium metal battery through a silicon-based superionic conductor. J. Electrochem. Soc. 161, A1812–A1817 (2014).
Hoshina, K., Dokko, K. & Kanamura, K. Investigation on electrochemical interface between Li4Ti5O12 and Li1+xAlxTi2−x(PO4)3 NASICON-type solid electrolyte. J. Electrochem. Soc. 152, A2138–A2142 (2005).
Ohta, S. et al. Co-sinterable lithium garnet-type oxide electrolyte with cathode for all-solid-state lithium ion battery. J. Power Sources 265, 40–44 (2014).
Tatsumisago, M., Nagao, M. & Hayashi, A. Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries. J. Asian Ceram. Soc. 1, 17–25 (2013).
Ohta, N. et al. Enhancement of the high-rate capability of solid-state lithium batteries by nanoscale interfacial modification. Adv. Mater. 18, 2226–2229 (2006).
Ohta, N. et al. LiNbO3-coated LiCoO2 as cathode material for all solid-state lithium secondary batteries. Electrochem. Commun. 9, 1486–1490 (2007).
Takada, K. et al. Interfacial modification for high-power solid-state lithium batteries. Solid State Ion. 179, 1333–1337 (2008).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Cheng, L. et al. The origin of high electrolyte–electrode interfacial resistances in lithium cells containing garnet type solid electrolytes. Phys. Chem. Chem. Phys. 16, 18294–18300 (2014).
Han, X. et al. Negating interfacial impedance in garnet-based solid-state Li metal batteries. Nat. Mater. 16, 572–579 (2017).
Wenzel, S., Leichtweiss, T., Krüger, D., Sann, J. & Janek, J. Interphase formation on lithium solid electrolytes—an in situ approach to study interfacial reactions by photoelectron spectroscopy. Solid State Ion. 278, 98–105 (2015).
Richards, W. D., Miara, L. J., Wang, Y., Kim, J. C. & Ceder, G. Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016).
Zhu, Y., He, X. & Mo, Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A 4, 3253–3266 (2016).
Zhu, Y., He, X. & Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Xiao, Y., Miara, Lincoln, J., Wang, Y. & Ceder, G. Computational screening of cathode coatings for solid-state batteries. Joule 3, 1252–1275 (2019).
Koerver, R. et al. Redox-active cathode interphases in solid-state batteries. J. Mater. Chem. A 5, 22750–22760 (2017).
Yoon, K., Kim, J.-J., Seong, W. M., Lee, M. H. & Kang, K. Investigation on the interface between Li10GeP2S12 electrolyte and carbon conductive agents in all-solid-state lithium battery. Sci. Rep. 8, 8066 (2018).
Zhang, W. et al. The detrimental effects of carbon additives in Li10GeP2S12-based solid-state batteries. ACS Appl. Mater. Interfaces 9, 35888–35896 (2017).
Xu, L. et al. Interfaces in solid-state lithium batteries. Joule 2, 1991–2015 (2018).
Brazier, A. et al. First cross-section observation of an all solid-state lithium-ion “nanobattery” by transmission electron microscopy. Chem. Mater. 20, 2352–2359 (2008).
Schwöbel, A., Hausbrand, R. & Jaegermann, W. Interface reactions between LiPON and lithium studied by in-situ X-ray photoemission. Solid State Ion. 273, 51–54 (2015).
Aydinol, M. K., Kohan, A. F., Ceder, G., Cho, K. & Joannopoulos, J. Ab initio study of lithium intercalation in metal oxides and metal dichalcogenides. Phys. Rev. B 56, 1354–1365 (1997).
Klein, F., Jache, B., Bhide, A. & Adelhelm, P. Conversion reactions for sodium-ion batteries. Phys. Chem. Chem. Phys. 15, 15876–15887 (2013).
Wang, F. et al. Conversion reaction mechanisms in lithium ion batteries: study of the binary metal fluoride electrodes. J. Am. Chem. Soc. 133, 18828–18836 (2011).
Urban, A., Seo, D.-H. & Ceder, G. Computational understanding of Li-ion batteries. npj Comput. Mater. 2, 16002 (2016).
Visbal, H. et al. The effect of diamond-like carbon coating on LiNi0.8Co0.15Al0.05O2 particles for all solid-state lithium-ion batteries based on Li2S–P2S5 glass-ceramics. J. Power Sources 314, 85–92 (2016).
Kim, K. H. et al. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery. J. Power Sources 196, 764–767 (2011).
Miara, L. et al. About the compatibility between high voltage spinel cathode materials and solid oxide electrolytes as a function of temperature. ACS Appl. Mater. Interfaces 8, 26842–26850 (2016).
Zarabian, M., Bartolini, M., Pereira-Almao, P. & Thangadurai, V. X-ray photoelectron spectroscopy and AC impedance spectroscopy studies of Li-La-Zr-O solid electrolyte thin film/LiCoO2 cathode interface for all-solid-state Li batteries. J. Electrochem. Soc. 164, A1133–A1139 (2017).
Appapillai, A. T., Mansour, A. N., Cho, J. & Shao-Horn, Y. Microstructure of LiCoO2 with and without “AlPO4” nanoparticle coating: combined STEM and XPS studies. Chem. Mater. 19, 5748–5757 (2007).
Jain, A. et al. Commentary: The Materials Project: a materials genome approach to accelerating materials innovation. APL Mater. 1, 011002 (2013).
Lepley, N. D. & Holzwarth, N. A. W. Modeling interfaces between solids: application to Li battery materials. Phys. Rev. B 92, 1–15 (2015).
Sharafi, A. et al. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem. Mater. 29, 7961–7968 (2017).
Haruyama, J., Sodeyama, K. & Tateyama, Y. Cation mixing properties toward Co diffusion at the LiCoO2 cathode/sulfide electrolyte interface in a solid-state battery. ACS Appl. Mater. Interfaces 9, 286–292 (2016).
Xu, Z.-M., Bo, S.-H. & Zhu, H. LiCrS2 and LiMnS2 cathodes with extraordinary mixed electron-ion conductivities and favorable interfacial compatibilities with sulfide electrolyte. ACS Appl. Mater. Interfaces 10, 36941–36953 (2018).
Sicolo, S., Fingerle, M., Hausbrand, R. & Albe, K. Interfacial instability of amorphous LiPON against lithium: a combined density functional theory and spectroscopic study. J. Power Sources 354, 124–133 (2017).
Sumita, M., Tanaka, Y., Ikeda, M. & Ohno, T. Charged and discharged states of cathode/sulfide electrolyte interfaces in all-solid-state lithium ion batteries. J. Phys. Chem. C 120, 13332–13339 (2016).
Camacho-Forero, L. E. & Balbuena, P. B. Exploring interfacial stability of solid-state electrolytes at the lithium-metal anode surface. J. Power Sources 396, 782–790 (2018).
Tang, H. et al. Probing solid–solid interfacial reactions in all-solid-state sodium-ion batteries with first-principles calculations. Chem. Mater. 30, 163–173 (2017).
Liu, Z. et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4. J. Am. Chem. Soc. 135, 975–978 (2013).
Hayashi, A., Muramatsu, H., Ohtomo, T., Hama, S. & Tatsumisago, M. Improvement of chemical stability of Li3PS4 glass electrolytes by adding MxOy (M = Fe, Zn, and Bi) nanoparticles. J. Mater. Chem. A 1, 6320–6326 (2013).
Oh, G., Hirayama, M., Kwon, O., Suzuki, K. & Kanno, R. Bulk-type all solid-state batteries with 5 V class LiNi0.5Mn1.5O4 cathode and Li10GeP2S12 solid electrolyte. Chem. Mater. 28, 2634–2640 (2016).
Chu, I.-H. et al. Insights into the performance limits of the Li7P3S11 superionic conductor: a combined first-principles and experimental study. ACS Appl. Mater. Interfaces 8, 7843–7853 (2016).
Mo, Y., Ong, S. P. & Ceder, G. First principles study of the Li10GeP2S12 lithium super ionic conductor material. Chem. Mater. 24, 15–17 (2012).
Han, F., Zhu, Y., He, X., Mo, Y. & Wang, C. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes. Adv. Energy Mater. 6, 1–9 (2016).
Han, F., Gao, T., Zhu, Y., Gaskell, K. J. & Wang, C. A battery made from a single material. Adv. Mater. 27, 3473–3483 (2015).
Wu, X., Villevieille, C., Novák, P. & El Kazzi, M. Monitoring the chemical and electronic properties of electrolyte–electrode interfaces in all-solid-state batteries using operando X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 20, 11123–11129 (2018).
Bron, P. et al. Li10SnP2S12: an affordable lithium superionic conductor. J. Am. Chem. Soc. 135, 15694–15697 (2013).
Tarhouchi, I., Viallet, V., Vinatier, P. & Ménétrier, M. Electrochemical characterization of Li10SnP2S12: an electrolyte or a negative electrode for solid state Li-ion batteries? Solid State Ion. 296, 18–25 (2016).
Hakari, T. et al. Structural and electronic-state changes of a sulfide solid electrolyte during the Li deinsertion–insertion processes. Chem. Mater. 29, 4768–4774 (2017).
Swamy, T., Chen, X. & Chiang, Y.-M. Electrochemical redox behavior of Li-ion conducting sulfide solid electrolytes. Chem. Mater. 31, 707–713 (2019).
Zhang, W. et al. Degradation mechanisms at the Li10GeP2S12/LiCoO2 cathode interface in an all-solid-state lithium ion battery. ACS Appl. Mater. Interfaces 10, 22226–22236 (2018).
Lacivita, V., Wang, Y., Bo, S.-H. & Ceder, G. Ab initio investigation of the stability of electrolyte/electrode interfaces in all-solid-state Na batteries. J. Mater. Chem. A 7, 8144–8155 (2019).
Dean, J. A. Lange’s Handbook of Chemistry (McGraw-Hill, 1999).
Tsukasaki, H. et al. Exothermal mechanisms in the charged LiNi1/3Mn1/3Co1/3O2 electrode layers for sulfide-based all-solid-state lithium batteries. J. Power Sources 434, 226714 (2019).
Sang, L., Haasch, R. T., Gewirth, A. A. & Nuzzo, R. G. Evolution at the solid electrolyte/gold electrode interface during lithium deposition and stripping. Chem. Mater. 29, 3029–3037 (2017).
Wenzel, S. et al. Interphase formation and degradation of charge transfer kinetics between a lithium metal anode and highly crystalline Li7P3S11 solid electrolyte. Solid State Ion. 286, 24–33 (2016).
Choi, S.-J. et al. Synthesis and electrochemical characterization of a glass-ceramic Li7P2S8I solid electrolyte for all-solid-state Li-ion batteries. J. Electrochem. Soc. 165, A957–A962 (2018).
Bron, P., Roling, B. & Dehnen, S. Impedance characterization reveals mixed conducting interphases between sulfidic superionic conductors and lithium metal electrodes. J. Power Sources 352, 127–134 (2017).
Suyama, M., Kato, A., Sakuda, A., Hayashi, A. & Tatsumisago, M. Lithium dissolution/deposition behavior with Li3PS4-LiI electrolyte for all-solid-state batteries operating at high temperatures. Electrochim. Acta 286, 158–162 (2018).
Wu, E. A. et al. New insights into the interphase between the Na metal anode and sulfide solid-state electrolytes: a joint experimental and computational study. ACS Appl. Mater. Interfaces 10, 10076–10086 (2018).
Tian, Y. et al. Reactivity-guided interface design in Na metal solid-state batteries. Joule 3, 1037–1050 (2019).
Deng, Z., Zhu, Z., Chu, I.-H. & Ong, S. P. Data-driven first-principles methods for the study and design of alkali superionic conductors. Chem. Mater. 29, 281–288 (2017).
Auvergniot, J. et al. Redox activity of argyrodite Li6PS5Cl electrolyte in all-solid-state Li-ion battery: an XPS study. Solid State Ion. 300, 78–85 (2017).
Auvergniot, J. et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries. Chem. Mater. 29, 3883–3890 (2017).
Wenzel, S. et al. Interfacial reactivity and interphase growth of argyrodite solid electrolytes at lithium metal electrodes. Solid State Ion. 318, 102–112 (2018).
Walther, F. et al. Visualization of the interfacial decomposition of composite cathodes in argyrodite based all-solid-state batteries using time-of-flight secondary ion mass spectrometry. Chem. Mater. 31, 3745–3755 (2019).
Zhang, Z. et al. All-in-one improvement toward Li6PS5Br-based solid electrolytes triggered by compositional tune. J. Power Sources 410, 162–170 (2019).
Garca-Martn, S., Amador, U., Morata-Orrantia, A., Rodrguez-Carvajal, J. & Alario-Franco, M. Á. Structure, microstructure, composition and properties of lanthanum lithium titanates and some substituted analogues. Z. Anorg. Allg. Chem. 635, 2363–2373 (2009).
Ma, C. et al. Atomic-scale origin of the large grain-boundary resistance in perovskite Li-ion-conducting solid electrolytes. Energy Environ. Sci. 7, 1638–1642 (2014).
Xu, X., Wen, Z., Wu, J. & Yang, X. Preparation and electrical properties of NASICON-type structured Li1.4Al0.4Ti1.6(PO4)3 glass-ceramics by the citric acid-assisted sol–gel method. Solid State Ion. 178, 29–34 (2007).
Li, S. et al. Reaction mechanism studies towards effective fabrication of lithium-rich anti-perovskites Li3OX (X = Cl, Br). Solid State Ion. 284, 14–19 (2016).
Inaguma, Y. & Nakashima, M. A rechargeable lithium–air battery using a lithium ion-conducting lanthanum lithium titanate ceramics as an electrolyte separator. J. Power Sources 228, 250–255 (2013).
Schipper, F. et al. Recent advances and remaining challenges for lithium ion battery cathodes I. Nickel-Rich, LiNixCoyMnzO2. J. Electrochem. Soc. 164, A6220–A6228 (2017).
Antolini, E. & Ferretti, M. Synthesis and thermal stability of LiCoO2. J. Solid State Chem. 117, 1–7 (1995).
Park, K. et al. Electrochemical nature of the cathode interface for a solid-state lithium-ion battery: interface between LiCoO2 and garnet-Li7La3Zr2O12. Chem. Mater. 28, 8051–8059 (2016).
Yoshinari, T. et al. Interfacial stability of phosphate-NASICON solid electrolytes in Ni-rich NCM cathode-based solid-state batteries. ACS Appl. Mater. Interfaces 11, 23244–23253 (2019).
Yu, S. et al. Monolithic all-phosphate solid-state lithium-ion battery with improved interfacial compatibility. ACS Appl. Mater. Interfaces 10, 22264–22277 (2018).
Qin, S. et al. Growth of self-textured Ga3+-substituted Li7La3Zr2O12 ceramics by solid state reaction and their significant enhancement in ionic conductivity. Appl. Phys. Lett. 112, 113901 (2018).
Murugan, R., Ramakumar, S. & Janani, N. High conductive yttrium doped Li7La3Zr2O12 cubic lithium garnet. Electrochem. Comm. 13, 1373–1375 (2011).
Murugan, R., Weppner, W., Schmid-Beurmann, P. & Thangadurai, V. Structure and lithium ion conductivity of bismuth containing lithium garnets Li5La3Bi2O12 and Li6SrLa2Bi2O12. Mater. Sci. Eng. B 143, 14–20 (2007).
Thangadurai, V., Kaack, H. & Weppner, W. J. F. Novel fast lithium ion conduction in garnet-type Li5La3M2O2 (M = Nb, Ta). J. Am. Ceram. Soc. 86, 437–440 (2003).
Li, Y., Han, J.-T., Wang, C.-A., Xie, H. & Goodenough, J. B. Optimizing Li+ conductivity in a garnet framework. J. Mater. Chem. 22, 15357–15361 (2012).
Thangadurai, V. & Weppner, W. Li6ALa2Ta2O12 (A=Sr, Ba): novel garnet-like oxides for fast lithium ion conduction. Adv. Funct. Mater. 15, 107–112 (2005).
Li, Y. et al. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 140, 6448–6455 (2018).
Sharafi, A. et al. Impact of air exposure and surface chemistry on Li–Li7La3Zr2O12 interfacial resistance. J. Mater. Chem. A 5, 13475–13487 (2017).
Dai, J., Yang, C., Wang, C., Pastel, G. & Hu, L. Interface engineering for garnet-based solid-state lithium-metal batteries: materials, structures, and characterization. Adv. Mater. 30, 1802068 (2018).
Hofstetter, K., Samson, A. J., Narayanan, S. & Thangadurai, V. Present understanding of the stability of Li-stuffed garnets with moisture, carbon dioxide, and metallic lithium. J. Power Sources 390, 297–312 (2018).
Kotobuki, M. & Kanamura, K. Fabrication of all-solid-state battery using Li5La3Ta2O12 ceramic electrolyte. Ceram. Int. 39, 6481–6487 (2013).
Kotobuki, M., Kanamura, K., Sato, Y. & Yoshida, T. Fabrication of all-solid-state lithium battery with lithium metal anode using Al2O3-added Li7La3Zr2O12 solid electrolyte. J. Power Sources 196, 7750–7754 (2011).
Rangasamy, E. et al. A high conductivity oxide–sulfide composite lithium superionic conductor. J. Mater. Chem. A 2, 4111–4116 (2014).
Jalem, R. et al. Experimental and first-principles DFT study on the electrochemical reactivity of garnet-type solid electrolytes with carbon. J. Mater. Chem. A 4, 14371–14379 (2016).
Miara, L. J., Richards, W. D., Wang, Y. E. & Ceder, G. First-principles studies on cation dopants and electrolyte|cathode interphases for lithium garnets. Chem. Mater. 27, 4040–4047 (2015).
Nakayama, M., Kotobuki, M., Munakata, H., Nogami, M. & Kanamura, K. First-principles density functional calculation of electrochemical stability of fast Li ion conducting garnet-type oxides. Phys. Chem. Chem. Phys. 14, 10008–10014 (2012).
Li, Y., Wang, C.-A., Xie, H., Cheng, J. & Goodenough, J. B. High lithium ion conduction in garnet-type Li6La3ZrTaO12. Electrochem. Comm. 13, 1289–1292 (2011).
Nemori, H. et al. Stability of garnet-type solid electrolyte LixLa3A2−yByO12 (A = Nb or Ta, B = Sc or Zr). Solid State Ion. 282, 7–12 (2015).
Kim, Y. et al. Electrochemical stability of Li6.5La3Zr1.5M0.5O12 (M= Nb or Ta) against metallic lithium. Front Energy Res. 4, 20 (2016).
Cheng, L. et al. Effect of microstructure and surface impurity segregation on the electrical and electrochemical properties of dense Al-substituted Li7La3Zr2O12. J. Mater. Chem. A 2, 172–181 (2014).
Yan, X. et al. A novel thin solid electrolyte film and its application in all-solid-state battery at room temperature. Ionics 24, 1545–1551 (2018).
Fingerle, M., Loho, C., Ferber, T., Hahn, H. & Hausbrand, R. Evidence of the chemical stability of the garnet-type solid electrolyte Li5La3Ta2O12 towards lithium by a surface science approach. J. Power Sources 366, 72–79 (2017).
Wolfenstine, J., Allen, J. L., Read, J. & Sakamoto, J. Chemical stability of cubic Li7La3Zr2O12 with molten lithium at elevated temperature. J. Mater. Sci. 48, 5846–5851 (2013).
Ma, C., Cheng, Y., Yin, K., Luo, J. & Sharafi, A. Interfacial stability of Li metal-solid electrolyte elucidated via in situ electron microscopy. Nano Lett. 16, 7030–7036 (2016).
Zhu, Y. et al. Dopant-dependent stability of garnet solid electrolyte interfaces with lithium metal. Adv. Energy Mater. 9, (1803440 (2019).
Rettenwander, D. et al. Interface instability of Fe-stabilized Li7La3Zr2O12 versus Li metal. J. Phys. Chem. C 122, 3780–3785 (2018).
Afyon, S., Krumeich, F. & Rupp, J. L. M. A shortcut to garnet-type fast Li-ion conductors for all-solid state batteries. J. Mater. Chem. A 3, 18636–18648 (2015).
Ong, S. P., Wang, L., Kang, B. & Ceder, G. Li–Fe–P–O2 phase diagram from first principles calculations. Chem. Mater. 20, 1798–1807 (2008).
Yi, E., Wang, W., Kieffer, J. & Laine, R. M. Flame made nanoparticles permit processing of dense, flexible, Li+ conducting ceramic electrolyte thin films of cubic-Li7La3Zr2O12 (c-LLZO). J. Mater. Chem. A 4, 12947–12954 (2016).
Ren, Y., Liu, T., Shen, Y., Lin, Y. & Nan, C.-W. Chemical compatibility between garnet-like solid state electrolyte Li6.75La3Zr1.75Ta0.25O12 and major commercial lithium battery cathode materials. J. Materiomics 2, 256–264 (2016).
Thangadurai, V. & Weppner, W. Investigations on electrical conductivity and chemical compatibility between fast lithium ion conducting garnet-like Li6BaLa2Ta2O12 and lithium battery cathodes. J. Power Sources 142, 339–344 (2005).
Tsai, C.-L. et al. A garnet structure-based all-solid-state Li battery without interface modification: resolving incompatibility issues on positive electrodes. Sustain. Energy Fuels 3, 280–291 (2019).
Bitzer, M., Van Gestel, T. & Uhlenbruck, S. Sol-gel synthesis of thin solid Li7La3Zr2O12 electrolyte films for Li-ion batteries. Thin Solid Films 615, 128–134 (2016).
Vardar, G. et al. Structure, chemistry, and charge transfer resistance of the interface between Li7La3Zr2O12 electrolyte and LiCoO2 cathode. Chem. Mater. 30, 6259–6276 (2018).
Ellis, B. L., Lee, K. T. & Nazar, L. F. Positive electrode materials for Li-ion and Li-batteries. Chem. Mater. 22, 691–714 (2010).
Ohta, S., Kobayashi, T., Seki, J. & Asaoka, T. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 202, 332–335 (2012).
Ma, X., Kang, B. & Ceder, G. High rate micron-sized ordered LiNi0.5Mn1.5O4. J. Electrochem. Soc. 157, A925–A931 (2010).
Manthiram, A., Chemelewski, K. & Lee, E.-S. A perspective on the high-voltage LiMn1.5Ni0.5O4 spinel cathode for lithium-ion batteries. Energy Environ. Sci. 7, 1339–1350 (2014).
Bates, J. B. et al. Fabrication and characterization of amorphous lithium electrolyte thin films and rechargeable thin-film batteries. J. Power Sources 43, 103–110 (1993).
Suzuki, N., Shirai, S., Takahashi, N., Inaba, T. & Shiga, T. A lithium phosphorous oxynitride (LiPON) film sputtered from unsintered Li3PO4 powder target. Solid State Ion. 191, 49–54 (2011).
Le Van-Jodin, L., Ducroquet, F., Sabary, F. & Chevalier, I. Dielectric properties, conductivity and Li+ ion motion in LiPON thin films. Solid State Ion. 253, 151–156 (2013).
Li, J., Dudney, N. J., Nanda, J. & Liang, C. Artificial solid electrolyte interphase to address the electrochemical degradation of silicon electrodes. ACS Appl. Mater. Interfaces 6, 10083–10088 (2014).
Bates, J. B., Dudney, N. J., Neudecker, B., Ueda, A. & Evans, C. D. Thin-film lithium and lithium-ion batteries. Solid State Ion. 135, 33–45 (2000).
Alpen, U. V., Rabenau, A. & Talat, G. H. Ionic conductivity in Li3N single crystals. Appl. Phys. Lett. 30, 621–623 (1977).
Nazri, G. Preparation, structure and ionic conductivity of lithium phosphide. Solid State Ion. 34, 97–102 (1989).
Fingerle, M., Buchheit, R., Sicolo, S., Albe, K. & Hausbrand, R. Reaction and space charge layer formation at the LiCoO2–LiPON interface: insights on defect formation and ion energy level alignment by a combined surface science–simulation approach. Chem. Mater. 29, 7675–7685 (2017).
Wang, Z. et al. In situ STEM-EELS observation of nanoscale interfacial phenomena in all-solid-state batteries. Nano Lett. 16, 3760–3767 (2016).
Inaguma, Y. et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 86, 689–693 (1993).
Chen, C. H. & Amine, K. Ionic conductivity, lithium insertion and extraction of lanthanum lithium titanate. Solid State Ion. 144, 51–57 (2001).
Bohnke, O., Bohnke, Cl & Fourquet, J. L. Mechanism of ionic conduction and electrochemical intercalation of lithium into the perovskite lanthanum lithium titanate. Solid State Ion. 91, 21–31 (1996).
Shan, Y. J., Chen, L., Inaguma, Y., Itoh, M. & Nakamura, T. Oxide cathode with perovskite structure for rechargeable lithium batteries. J. Power Sources 54, 397–402 (1995).
Jiang, Z. et al. Perovskite membranes with vertically aligned microchannels for all-solid-state lithium batteries. Adv. Energy Mater. 8, 1801433 (2018).
Nakayama, M., Usui, T., Uchimoto, Y., Wakihara, M. & Yamamoto, M. Changes in electronic structure upon lithium insertion into the A-site deficient perovskite type oxides (Li, La) TiO3. J. Phys. Chem. B 109, 4135–4143 (2005).
Kishida, K. et al. Microstructure of the LiCoO2 (cathode)/La2/3−xLi3xTiO3 (electrolyte) interface and its influences on the electrochemical properties. Acta Mater. 55, 4713–4722 (2007).
Liao, C.-L., Wen, C.-H. & Fung, K.-Z. The stability between perovskite La2/3−xLi3x□1/3−2xTiO3 (3x = 0.3) electrolyte and LiMmOn (M = Mn, Ni and Co) cathodes. J. Alloys Compd. 432, L22–L25 (2007).
Kotobuki, M. et al. Compatibility of LiCoO2 and LiMn2O4 cathode materials for Li0.55La0.35TiO3 electrolyte to fabricate all-solid-state lithium battery. J. Power Sources 195, 5784–5788 (2010).
Lü, X. et al. Antiperovskite Li3OCl superionic conductor films for solid-state Li-ion batteries. Adv. Sci. 3, 1500359 (2016).
Lü, X. et al. Li-rich anti-perovskite Li3OCl films with enhanced ionic conductivity. Chem. Commun. 50, 11520–11522 (2014).
Emly, A., Kioupakis, E. & Van der Ven, A. Phase stability and transport mechanisms in antiperovskite Li3OCl and Li3OBr superionic conductors. Chem. Mater. 25, 4663–4670 (2013).
Zhang, Y., Zhao, Y. & Chen, C. Ab initio study of the stabilities of and mechanism of superionic transport in lithium-rich antiperovskites. Phys. Rev. B 87, 134303 (2013).
Whangbo, M.-H., Koo, H.-J., Villesuzanne, A. & Pouchard, M. Effect of metal–oxygen covalent bonding on the competition between Jahn–Teller distortion and charge disproportionation in the perovskites of high-spin d4 metal ions LaMnO3 and CaFeO3. Inorg. Chem. 41, 1920–1929 (2002).
Braga, M. H., Ferreira, J. A., Stockhausen, V., Oliveira, J. E. & El-Azab, A. Novel Li3ClO based glasses with superionic properties for lithium batteries. J. Mater. Chem. A 2, 5470–5480 (2014).
Li, Y. et al. Fluorine-doped antiperovskite electrolyte for all-solid-state lithium-ion batteries. Angew. Chem. Int. Ed. 55, 9965–9968 (2016).
Anantharamulu, N. et al. A wide-ranging review on Nasicon type materials. J. Mater. Sci. 46, 2821–2837 (2011).
Arbi, K., Bucheli, W., Jiménez, R. & Sanz, J. High lithium ion conducting solid electrolytes based on NASICON Li1+xAlxM2−x(PO4)3 materials (M = Ti, Ge and 0 ≤ x ≤ 0.5). J. Eur. Ceram. Soc. 35, 1477–1484 (2015).
Fu, J. Fast Li+ ion conducting glass-ceramics in the system Li2O–Al2O3–GeO2–P2O5. Solid State Ion. 104, 191–194 (1997).
Feng, J. K., Lu, L. & Lai, M. O. Lithium storage capability of lithium ion conductor Li1.5Al0.5Ge1.5(PO4)3. J. Alloys Compd. 501, 255–258 (2010).
Hartmann, P. et al. Degradation of NASICON-type materials in contact with lithium metal: formation of mixed conducting interphases (MCI) on solid electrolytes. J. Phys. Chem. C 117, 21064–21074 (2013).
Wu, B. et al. The role of the solid electrolyte interphase layer in preventing Li dendrite growth in solid-state batteries. Energy Environ. Sci. 11, 1803–1810 (2018).
He, L. et al. Failure mechanism and interface engineering for NASICON structure all-solid-state lithium metal batteries. ACS. Appl. Mater. Interfaces 11, 20895–20904 (2019).
Lewis, J. A. et al. Interphase morphology between a solid-state electrolyte and lithium controls cell failure. ACS Energy Lett. 4, 591–599 (2019).
Tippens, J. et al. Visualizing chemo-mechanical degradation of a solid-state battery electrolyte. ACS Energy Lett. 4, 1475–1483 (2019).
Xu, X., Wen, Z., Wu, X., Yang, X. & Gu, Z. Lithium ion-conducting glass-ceramics of Li1.5Al0.5Ge1.5(PO4)3−xLi2O (x=0.0–0.20) with good electrical and electrochemical properties. J. Am. Ceram. Soc. 90, 2802–2806 (2007).
Kim, H.-S. et al. Characterization of sputter-deposited LiCoO2 thin film grown on NASICON-type electrolyte for application in all-solid-state rechargeable lithium battery. ACS Appl. Mater. Interfaces 9, 16063–16070 (2017).
Li, Y. et al. Mastering the interface for advanced all-solid-state lithium rechargeable batteries. Proc. Natl Acad. Sci. USA 113, 13313–13317 (2016).
El-Shinawi, H., Regoutz, A., Payne, D. J., Cussen, E. J. & Corr, S. A. NASICON LiM2(PO4)3 electrolyte (M= Zr) and electrode (M= Ti) materials for all solid-state Li-ion batteries with high total conductivity and low interfacial resistance. J. Mater. Chem. A 6, 5296–5303 (2018).
Kato, T. et al. In-situ Li7La3Zr2O12/LiCoO2 interface modification for advanced all-solid-state battery. J. Power Sources 260, 292–298 (2014).
Kim, Y. et al. High voltage stability of LiCoO2 particles with a nano-scale Lipon coating. Electrochim. Acta 56, 6573–6580 (2011).
Martha, S. K., Nanda, J., Kim, Y., Unocic, R. R. & Pannala, S. Solid electrolyte coated high voltage layered-layered lithium-rich composite cathode: Li1.2Mn0.525Ni0.175Co0.1O2. J. Mater. Chem. A 1, 5587–5595 (2013).
Song, J., Jacke, S., Becker, D., Hausbrand, R. & Jaegermann, W. Stabilization of thin film LiCoO2 electrode by LiPON coating. Electrochem. Solid-State Lett. 14, A11–A13 (2011).
Li, X., et al. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 40:39-45.
Ito, S. et al. A rocking chair type all-solid-state lithium ion battery adopting Li2O–ZrO2 coated LiNi0.8Co0.15Al0.05O2 and a sulfide based electrolyte. J. Power Sources 248, 943–950 (2014).
Sakuda, A., Kitaura, H., Hayashi, A., Tadanaga, K. & Tatsumisago, M. Improvement of high-rate performance of all-solid-state lithium secondary batteries using LiCoO2 coated with Li2O–SiO2 glasses. Electrochem. Solid-State Lett. 11, A1–A3 (2008).
Sakurai, Y., Sakuda, A., Hayashi, A. & Tatsumisago, M. Preparation of amorphous Li4SiO4–Li3PO4 thin films by pulsed laser deposition for all-solid-state lithium secondary batteries. Solid State Ion. 182, 59–63 (2011).
Jung, S. H. et al. Li3BO3–Li2CO3: rationally designed buffering phase for sulfide all-solid-state Li-ion batteries. Chem. Mater. 30, 8190–8200 (2018).
Chen, K. et al. Effect of introducing interlayers into electrode/electrolyte interface in all-solid-state battery using sulfide electrolyte. Solid State Ion. 327, 150–156 (2018).
Kwak, H. W. & Park, Y. J. Cathode coating using LiInO2-LiI composite for stable sulfide-based all-solid-state batteries. Sci. Rep. 9, 8099 (2019).
Kwak, H. W. & Park, Y. J. Li2MoO4 coated Ni-rich cathode for all-solid-state batteries. Thin Solid Films 660, 625–630 (2018).
Ohta, S. et al. All-solid-state lithium ion battery using garnet-type oxide and Li3BO3 solid electrolytes fabricated by screen-printing. J. Power Sources 238, 53–56 (2013).
Liu, Y. et al. Stabilizing the interface of NASICON solid electrolyte against Li metal with atomic layer deposition. ACS Appl. Mater. Interfaces 10, 31240–31248 (2018).
Sang, L. et al. Understanding the effect of interlayers at the thiophosphate solid electrolyte/lithium interface for all-solid-state Li batteries. Chem. Mater. 30, 8747–8756 (2018).
Ruan, Y. et al. Acid induced conversion towards robust and lithiophilic interface for Li-Li7La3Zr2O12 solid-state battery. J. Mater. Chem. A 7, 14565–14574 (2019).
Zhang, Z. et al. Interface re-engineering of Li10GeP2S12 electrolyte and lithium anode for all-solid-state lithium batteries with ultralong cycle life. ACS Appl. Mater. Interfaces 10, 2556–2565 (2018).
Zhu, Y., He, X. & Mo, Y. Strategies based on nitride materials chemistry to stabilize Li metal anode. Adv. Sci. 4, 1600517 (2017).
Cheng, Q. et al. Stabilizing solid electrolyte-anode interface in Li-metal batteries by boron nitride-based nanocomposite coating. Joule 3, 1510–1522 (2019).
Shigeno, M. et al. New lithium-conducting nitride glass Li3BN2. Solid State Ion. 339, 114985 (2019).
Wang, Y., Richards, W. D., Bo, S.-H., Miara, L. J. & Ceder, G. Computational prediction and evaluation of solid-state sodium superionic conductors Na7P3X11 (X = O, S, Se). Chem. Mater. 29, 7475–7482 (2017).
Muy, S. et al. Tuning mobility and stability of lithium ion conductors based on lattice dynamics. Energy Environ. Sci. 11, 850–859 (2018).
Wang, S. et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 58, 8039–8043 (2019).
Asano, T. et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 30, 1803075 (2018).
Li, X. et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 12, 2665–2671 (2019).
Krauskopf, T., Culver, S. P. & Zeier, W. G. Bottleneck of diffusion and inductive effects in Li10Ge1−xSnxP2S12. Chem. Mater. 30, 1791–1798 (2018).
Nolan, A. M., Zhu, Y., He, X., Bai, Q. & Mo, Y. Computation-accelerated design of materials and interfaces for all-solid-state lithium-ion batteries. Joule 2, 2016–2046 (2018).
Sendek, A. D. et al. Holistic computational structure screening of more than 12000 candidates for solid lithium-ion conductor materials. Energy Environ. Sci. 10, 306–320 (2017).
Pradel, A. & Ribes, M. Lithium chalcogenide conductive glasses. Mater. Chem. Phys. 23, 121–142 (1989).
Duchêne, L. et al. A highly stable sodium solid-state electrolyte based on a dodeca/deca-borate equimolar mixture. Chem. Commun. 53, 4195–4198 (2017).
Sadikin, Y., Brighi, M., Schouwink, P. & Cˇerny`, R. Superionic conduction of sodium and lithium in anion-mixed hydroborates Na3BH4B12H12 and (Li0.7Na0.3)3BH4B12H12. Adv. Energy Mater. 5, 1501016 (2015).
Dewald, G., et.al. Experimental assessment of the practical oxidative stability of lithium thiophosphate solid electrolytes. Chem. Mater., 31:8328-8337, 2019.
Hori, S. et al. Synthesis, structure, and ionic conductivity of solid solution, Li10+δM1+ δ P2− δS12 (M = Si, Sn). Faraday Discuss. 176, 83–94 (2015).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2017).
Yu, C. et al. Tailoring Li6PS5Br ionic conductivity and understanding of its role in cathode mixtures for high performance all-solid-state Li–S batteries. J. Mater. Chem. A 7, 10412–10421 (2019).
Smith, A. J., Burns, J. C. & Dahn, J. R. A high precision study of the Coulombic efficiency of Li-ion batteries. Electrochem. Solid-State Lett. 13, A177–A179 (2010).
Smith, A. J., Burns, J. C., Trussler, S. & Dahn, J. R. Precision measurements of the coulombic efficiency of lithium-ion batteries and of electrode materials for lithium-ion batteries. J. Electrochem. Soc. 157, A196–A202 (2010).
Farhad, S. & Nazari, A. Introducing the energy efficiency map of lithium-ion batteries. Int. J. Energy Res. 43, 931–944 (2019).
Meister, P. et al. Best practice: performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 28, 7203–7217 (2016).
Wang, Y. et al. Design principles for solid-state lithium superionic conductors. Nat. Mater. 14, 1026–1031 (2015).
Xiong, S. et al. Computation-guided design of LiTaSiO5, a new lithium ionic conductor with sphene structure. Adv. Energy Mater. 9, 1803821 (2019).
He, X., Zhu, Y. & Mo, Y. Origin of fast ion diffusion in super-ionic conductors. Nat. Commun. 8, 15893 (2017).
Curtarolo, S. et al. The high-throughput highway to computational materials design. Nat. Mater. 12, 191–201 (2013).
Jain, A. et al. A high-throughput infrastructure for density functional theory calculations. Comput. Mater. Sci. 50, 2295–2310 (2011).
Acknowledgements
The work on ionic conductivity design was funded by the Samsung Advanced Institute of Technology. The development of the interfacial reactivity theory was funded by the Materials Project Program (grant no. KC23MP) through the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, Materials Sciences and Engineering Division under contract no. DE-AC02-05CH11231. Some of the work on sulfide electrolytes was supported by the Assistant Secretary of Energy Efficiency and Renewable Energy, Vehicle Technologies Office of the U.S. Department of Energy under contract no. DE-AC02-05CH11231 under the Advanced Battery Materials Research (BMR) Program.
Author information
Authors and Affiliations
Contributions
G.C. conceived the manuscript. Y.X. researched the data. S.-H.B. and Y.X. wrote the section on sulfides. Y.X. wrote the sections on garnets and coatings. J.C.K. wrote the sections on LiPON and antiperovskites. Y.W. and Y.X. wrote the sections on perovskites and NASICONs. G.C., Y.X. and L.J.M. wrote the discussion and conclusions sections. Y.X., Y.W. and L.J.M. designed the table and figures. All authors edited and reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, Y., Wang, Y., Bo, SH. et al. Understanding interface stability in solid-state batteries. Nat Rev Mater 5, 105–126 (2020). https://doi.org/10.1038/s41578-019-0157-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41578-019-0157-5
This article is cited by
-
Unlocking Li superionic conductivity in face-centred cubic oxides via face-sharing configurations
Nature Materials (2024)
-
A lithium–tin fluoride anode enabled by ionic/electronic conductive paths for garnet-based solid-state lithium metal batteries
Rare Metals (2024)
-
Dielectric LiNbO3 electrolyte regulating internal electric field in composite solid-state electrolyte to fundamentally boost Li-ion transport
Science China Materials (2024)
-
Li–Solid Electrolyte Interfaces/Interphases in All-Solid-State Li Batteries
Electrochemical Energy Reviews (2024)
-
Highly Efficient Aligned Ion-Conducting Network and Interface Chemistries for Depolarized All-Solid-State Lithium Metal Batteries
Nano-Micro Letters (2024)