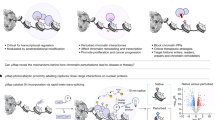
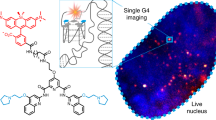
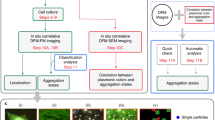
Abstract
Gold nanorods are one of the most widely explored inorganic materials in nanomedicine for diagnostics, therapeutics and sensing1. It has been shown that gold nanorods are not cytotoxic and localize within cytoplasmic vesicles following endocytosis, with no nuclear localization2,3, but other studies have reported alterations in gene expression profiles in cells following exposure to gold nanorods, via unknown mechanisms4. In this work we describe a pathway that can contribute to this phenomenon. By mapping the intracellular chemical speciation process of gold nanorods, we show that the commonly used Au–thiol conjugation, which is important for maintaining the noble (inert) properties of gold nanostructures, is altered following endocytosis, resulting in the formation of Au(i)–thiolates that localize in the nucleus5. Furthermore, we show that nuclear localization of the gold species perturbs the dynamic microenvironment within the nucleus and triggers alteration of gene expression in human cells. We demonstrate this using quantitative visualization of ubiquitous DNA G-quadruplex structures, which are sensitive to ionic imbalances, as an indicator of the formation of structural alterations in genomic DNA.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the plots within this paper and other findings of this study are available from the corresponding authors upon reasonable request.
References
Mieszawska, A. J., Mulder, W. J. M., Fayad, Z. A. & Cormode, D. P. Multifunctional gold nanoparticles for diagnosis and therapy of disease. Mol. Pharm. 10, 831–847 (2013).
Chithrani, B. D., Ghazani, A. A. & Chan, W. C. W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6, 662–668 (2006).
Qiu, Y. et al. Surface chemistry and aspect ratio mediated cellular uptake of Au nanorods. Biomaterials 31, 7606–7619 (2010).
Hauck, T. S., Ghazani, A. A. & Chan, W. C. W. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small 4, 153–159 (2008).
Reimers, J. R., Ford, M. J., Halder, A., Ulstrup, J. & Hush, N. S. Gold surfaces and nanoparticles are protected by Au(0)–thiyl species and are destroyed when Au(i)–thiolates form. Proc. Natl Acad. Sci. USA 113, E1424–E1433 (2016).
Vigderman, L., Manna, P. & Zubarev, E. R. Quantitative replacement of cetyl trimethylammonium bromide by cationic thiol ligands on the surface of gold nanorods and their extremely large uptake by cancer cells. Angew. Chem. Int. Ed. 51, 636–641 (2012).
Alkilany, A. M. et al. Cellular uptake and cytotoxicity of gold nanorods: molecular origin of cytotoxicity and surface effects. Small 5, 701–708 (2009).
Wang, L. et al. Surface chemistry of gold nanorods: origin of cell membrane damage and cytotoxicity. Nanoscale 5, 8384 (2013).
Railsback, J. G. et al. Weakly charged cationic nanoparticles induce DNA bending and strand separation. Adv. Mater. 24, 4261–4265 (2012).
Lankaš, F., Šponer, J., Hobza, P. & Langowski, J. Sequence-dependent elastic properties of DNA. J. Mol. Biol. 299, 695–709 (2000).
Packer, M. J., Dauncey, M. P. & Hunter, C. A. Sequence-dependent DNA structure: tetranucleotide conformational maps. J. Mol. Biol. 295, 85–103 (2000).
Bochman, M. L., Paeschke, K. & Zakian, V. A. DNA secondary structures: stability and function of G-quadruplex structures. Nat. Rev. Genet. 13, 770–780 (2012).
Lam, E. Y. N., Beraldi, D., Tannahill, D. & Balasubramanian, S. G-quadruplex structures are stable and detectable in human genomic DNA. Nat. Commun. 4, 1796 (2013).
David, A. P. et al. G-quadruplexes as novel cis-elements controlling transcription during embryonic development. Nucleic Acids Res. 44, 4163–4173 (2016).
Biffi, G., Tannahill, D., McCafferty, J. & Balasubramanian, S. Quantitative visualization of DNA G-quadruplex structures in human cells. Nat. Chem. 5, 182–186 (2013).
Brown, R. V., Danford, F. L., Gokhale, V., Hurley, L. H. & Brooks, T. A. Demonstration that drug-targeted down-regulation of MYC in non-Hodgkins lymphoma is directly mediated through the promoter G-quadruplex. J. Biol. Chem. 286, 41018–41027 (2011).
Rodríguez-Fernández, J., Pérez-Juste, J., Mulvaney, P. & Liz-Marzán, L. M. Spatially-directed oxidation of gold nanoparticles by Au(iii)−CTAB complexes. J. Phys. Chem. B 109, 14257–14261 (2005).
Casaletto, M. P., Longo, A., Martorana, A., Prestianni, A. & Venezia, A. M. XPS study of supported gold catalysts: the role of Au0 and Au + δ species as active sites. Surf. Interface Anal. 38, 215–218 (2006).
Zou, T., Lum, C. T., Lok, C.-N., Zhang, J.-J. & Che, C.-M. Chemical biology of anticancer gold(iii) and gold(i) complexes. Chem. Soc. Rev. 44, 8786–8801 (2015).
Campbell, N. H. & Neidle, S. in Interplay between Metal Ions and Nucleic Acids (eds Sigel, A., Sigel, H. & Sigel, R. K. O.) 119–134 (Springer, Dordrecht, 2012).
Gunduz, N., Ceylan, H., Guler, M. O. & Tekinay, A. B. Intracellular accumulation of gold nanoparticles leads to inhibition of macropinocytosis to reduce the endoplasmic reticulum stress. Sci. Rep. 7, 40493 (2017).
Sinnamon, J. R., Waddell, C. B., Nik, S., Chen, E. I. & Czaplinski, K. Hnrpab regulates neural development and neuron cell survival after glutamate stimulation. RNA 18, 704–719 (2012).
Dai, J., Chen, D., Jones, R. A., Hurley, L. H. & Yang, D. NMR solution structure of the major G-quadruplex structure formed in the human BCL2 promoter region. Nucleic Acids Res. 34, 5133–5144 (2006).
Siddiqui-Jain, A., Grand, C. L., Bearss, D. J. & Hurley, L. H. Direct evidence for a G-quadruplex in a promoter region and its targeting with a small molecule to repress c-MYC transcription. Proc. Natl Acad. Sci. USA 99, 11593–11598 (2002).
Zheng, K., Chen, Z., Hao, Y. & Tan, Z. Molecular crowding creates an essential environment for the formation of stable G-quadruplexes in long double-stranded DNA. Nucleic Acids Res. 38, 327–338 (2010).
Neidle, S. Quadruplex nucleic acids as novel therapeutic targets. J. Med. Chem. 59, 5987–6011 (2016).
Jana, N. R., Gearheart, L. & Murphy, C. J. Seeding growth for size control of 5−40 nm diameter gold nanoparticles. Langmuir 17, 6782–6786 (2001).
Ye, X., Zheng, C., Chen, J., Gao, Y. & Murray, C. B. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 13, 765–771 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Dhapola, P. & Chowdhury, S. QuadBase2: web server for multiplexed guanine quadruplex mining and visualization. Nucleic Acids Res. 44, W277–W283 (2016).
Wedlock, L. E. et al. NanoSIMS multi-element imaging reveals internalisation and nucleolar targeting for a highly-charged polynuclear platinum compound. Chem. Commun. 49, 6944–6946 (2013).
Acknowledgements
The authors thank S. Balasubramanian for his gift of pSANG10-3F-BG4. This research was supported by the Australian Research Council, the National Health and Medical Research Council and the Raine Medical Research Foundation for the Healy Research Collaboration Award. The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy, Characterisation & Analysis, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments, as well as the WA X-ray Surface Analysis Facility of the John de Laeter Centre, funded by the Australian Research Council LIEF grant LE120100026. J.A.Kr. acknowledges Cancer Council Western Australia for a PhD Top Up scholarship.
Author information
Authors and Affiliations
Contributions
N.M.S. and K.S.I. developed the hypothesis. D.H., J.A.Ke. and K.S.I designed the GNR intracellular evaluation experiments. N.M.S., L.H.H., J.A.Kr., D.H. and R.C. designed the G4 interaction experiments. C.W.E., D.H. and J.A.Kr. performed the cell viability and toxicity analysis. M.No. performed the MTAB synthesis. N.M.S., M.Ng., A.L.K., A.J.B. and C.S.B. carried out BG4 antibody production and purification. T.D.C., J.A.Kr. and J.-P.V. performed the XPS experiments. D.H., J.A.Kr., P.G., H.J. and M.R.K. performed the NanoSIMS experiments. D.H. and P.T. performed the qPCR experiments. A.M.M., M.S. and D.H. performed the nanoparticle synthesis and characterization. J.A.Kr. and C.W.E. performed ICP experiments. M.A. and M.F. performed TEM/NanoSIMS sample preparation and analysis. All authors contributed to writing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary Figures 1–9
Rights and permissions
About this article
Cite this article
Ho, D., Kretzmann, J.A., Norret, M. et al. Intracellular speciation of gold nanorods alters the conformational dynamics of genomic DNA. Nature Nanotech 13, 1148–1153 (2018). https://doi.org/10.1038/s41565-018-0272-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-018-0272-2
This article is cited by
-
Gold Nanorods-Based Photothermal Therapy: Interactions Between Biostructure, Nanomaterial, and Near-Infrared Irradiation
Nanoscale Research Letters (2022)