Abstract
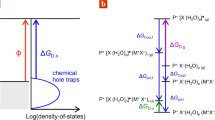
Doping of organic semiconductors is crucial for the operation of organic (opto)electronic and electrochemical devices. Typically, this is achieved by adding heterogeneous dopant molecules to the polymer bulk, often resulting in poor stability and performance due to dopant sublimation or aggregation. In small-molecule donor–acceptor systems, charge transfer can yield high and stable electrical conductivities, an approach not yet explored in all-conjugated polymer systems. Here, we report ground-state electron transfer in all-polymer donor–acceptor heterojunctions. Combining low-ionization-energy polymers with high-electron-affinity counterparts yields conducting interfaces with resistivity values five to six orders of magnitude lower than the separate single-layer polymers. The large decrease in resistivity originates from two parallel quasi-two-dimensional electron and hole distributions reaching a concentration of ∼1013 cm–2. Furthermore, we transfer the concept to three-dimensional bulk heterojunctions, displaying exceptional thermal stability due to the absence of molecular dopants. Our findings hold promise for electro-active composites of potential use in, for example, thermoelectrics and wearable electronics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors declare that the main data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data for Figs. 2–5 are provided with the paper.
References
Reineke, S., Thomschke, M., Lussem, B. & Leo, K. White organic light-emitting diodes: status and perspective. Rev. Mod. Phys. 85, 1245–1293 (2013).
Peumans, P., Yakimov, A. & Forrest, S. R. Small molecular weight organic thin-film photodetectors and solar cells. J. Appl. Phys. 93, 3693–3723 (2003).
Lussem, B. et al. Doped organic transistors. Chem. Rev. 116, 13714–13751 (2016).
Bubnova, O. & Crispin, X. Towards polymer-based organic thermoelectric generators. Energy Environ. Sci. 5, 9345–9362 (2012).
Walzer, K., Maennig, B., Pfeiffer, M. & Leo, K. Highly efficient organic devices based on electrically doped transport layers. Chem. Rev. 107, 1233–1271 (2007).
Bubnova, O. et al. Semi-metallic polymers. Nat. Mater. 13, 190–194 (2014).
Russ, B., Glaudell, A., Urban, J. J., Chabinyc, M. L. & Segalman, R. A. Organic thermoelectric materials for energy harvesting and temperature control. Nat. Rev. Mater. 1, 16050 (2016).
Salzmann, I., Heimel, G., Oehzelt, M., Winkler, S. & Koch, N. Molecular electrical doping of organic semiconductors: fundamental mechanisms and emerging dopant design rules. Acc. Chem. Res. 49, 370–378 (2016).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018).
Sun, H. et al. Complementary logic circuits based on high-performance n-type organic electrochemical transistors. Adv. Mater. 30, 1704916 (2018).
Nardes, A. M. et al. Microscopic understanding of the anisotropic conductivity of PEDOT: PSS thin films. Adv. Mater. 19, 1196–1200 (2007).
Qiu, L. et al. Enhancing doping efficiency by improving host-dopant miscibility for fullerene-based n-type thermoelectrics. J. Mater. Chem. A. 5, 21234–21241 (2017).
Pingel, P. & Neher, D. Comprehensive picture of p-type doping of P3HT with the molecular acceptor F4TCNQ. Phys. Rev. B. 87, 115209 (2013).
Zuo, G., Abdalla, H. & Kemerink, M. Impact of doping on the density of states and the mobility in organic semiconductors. Phys. Rev. B. 93, 235203 (2016).
Li, J. et al. Quantitative measurements of the temperature-dependent microscopic and macroscopic dynamics of a molecular dopant in a conjugated polymer. Macromolecules 50, 5476–5489 (2017).
Li, J. et al. Measurement of small molecular dopant F4TCNQ and C60F36 diffusion in organic bilayer architectures. ACS Appl. Mater. Interfaces 7, 28420–28428 (2015).
Hynynen, J. et al. Enhanced electrical conductivity of molecularly p-doped poly(3-hexylthiophene) through understanding the correlation with solid-state order. Macromolecules 50, 8140–8148 (2017).
Jacobs, I. E. & Moulé, A. J. Controlling molecular doping in organic semiconductors. Adv. Mater. 29, 1703063 (2017).
Kroon, R. et al. Polar side chains enhance processability, electrical conductivity, and thermal stability of a molecularly p-doped polythiophene. Adv. Mater. 29, 1700930 (2017).
Reiser, P. et al. Dopant diffusion in sequentially doped poly(3-hexylthiophene) studied by infrared and photoelectron spectroscopy. J. Phys. Chem. C. 122, 14518–14527 (2018).
Fahlman, M. et al. Interfaces in organic electronics. Nat. Rev. Mater. 4, 627–650 (2019).
Alves, H., Molinari, A. S., Xie, H. X. & Morpurgo, A. F. Metallic conduction at organic charge-transfer interfaces. Nat. Mater. 7, 574–580 (2008).
Odom, S. A. et al. Restoration of conductivity with TTF-TCNQ charge-transfer salts. Adv. Funct. Mater. 20, 1721–1727 (2010).
Hiraoka, M. et al. On-Substrate synthesis of molecular conductor films and circuits. Adv. Mater. 19, 3248–3251 (2007).
Jacobs, I. E. et al. Polymorphism controls the degree of charge transfer in a molecularly doped semiconducting polymer. Mater. Horiz. 5, 655–660 (2018).
Jérome, D. Organic conductors: from charge density wave TTF−TCNQ to superconducting (TMTSF)2PF6. Chem. Rev. 104, 5565–5592 (2004).
Chen, X. L. & Jenekhe, S. A. Bipolar conducting polymers: blends of p-type polypyrrole and an n-type ladder polymer. Macromolecules 30, 1728–1733 (1997).
Grancini, G. et al. Hot exciton dissociation in polymer solar cells. Nat. Mater. 12, 29–33 (2013).
Kawamura, Y., Yanagida, S. & Forrest, S. R. Energy transfer in polymer electrophosphorescent light emitting devices with single and multiple doped luminescent layers. J. Appl. Phys. 92, 87–93 (2002).
Yoshida, H. Principle and application of low energy inverse photoemission spectroscopy: a new method for measuring unoccupied states of organic semiconductors. J. Electron Spectrosc. Relat. Phenom. 204, 116–124 (2015).
Yoshida, H. Near-ultraviolet inverse photoemission spectroscopy using ultra-low energy electrons. Chem. Phys. Lett. 539-540, 180–185 (2012).
Oehzelt, M., Koch, N. & Heimel, G. Organic semiconductor density of states controls the energy level alignment at electrode interfaces. Nat. Commun. 5, 4174–4174 (2014).
Greiner, M. T. et al. Universal energy-level alignment of molecules on metal oxides. Nat. Mater. 11, 76–81 (2012).
Bokdam, M., Çakır, D. & Brocks, G. Fermi level pinning by integer charge transfer at electrode-organic semiconductor interfaces. Appl. Phys. Lett. 98, 113303–113303 (2011).
Braun, S., Salaneck, W. R. & Fahlman, M. Energy-level alignment at organic/metal and organic/organic interfaces. Adv. Mater. 21, 1450–1472 (2009).
Bao, Q., Braun, S., Wang, C., Liu, X. & Fahlman, M. Interfaces of (ultra)thin polymer films in organic electronics. Adv. Mater. Interfaces 6, 1800897 (2018).
Kiefer, D. et al. Double doping of conjugated polymers with monomer molecular dopants. Nat. Mater. 18, 149–155 (2019).
Pasveer, W. F. et al. Unified description of charge-carrier mobilities in disordered semiconducting polymers. Phys. Rev. Lett. 94, 206601 (2005).
Bässler, H. Charge transport in disordered organic photoconductors a Monte Carlo simulation study. Phys. Status Solidi B. 175, 15–56 (1993).
Zuo, G., Abdalla, H. & Kemerink, M. Conjugated polymer blends for organic thermoelectrics. Adv. Electron. Mater. 5, 1800821 (2019).
Zuo, G., Li, Z., Wang, E. & Kemerink, M. High seebeck coefficient and power factor in n-type organic thermoelectrics. Adv. Electron. Mater. 4, 1700501 (2018).
Melianas, A. et al. Photo-generated carriers lose energy during extraction from polymer-fullerene solar cells. Nat. Commun. 6, 8778 (2015).
Wang, S. et al. Thermoelectric properties of solution-processed n-doped ladder-type conducting polymers. Adv. Mater. 28, 10764–10771 (2016).
Fazzi, D., Fabiano, S., Ruoko, T.-P., Meerholz, K. & Negri, F. Polarons in π-conjugated ladder-type polymers: a broken symmetry density functional description. J. Mater. Chem. C. 7, 12876–12885 (2019).
Yao, C. J., Zhang, H. L. & Zhang, Q. Recent progress in thermoelectric materials based on conjugated polymers. Polymers 11, 107 (2019).
Arnold, F. E. & Van Deusen, R. L. Preparation and properties of high molecular weight, soluble oxobenz[de]imidazobenzimidazoisoquinoline ladder polymer. Macromolecules 2, 497–502 (1969).
Yoshida, H. Note: low energy inverse photoemission spectroscopy apparatus. Rev. Sci. Instrum. 85, 016101 (2014).
Jiang, Z. et al. The dedicated high-resolution grazing-incidence X-ray scattering beamline 8-ID-E at the advanced photon source. J. Synchrotron Radiat. 19, 627–636 (2012).
Jiang, Z. GIXSGUI: a MATLAB toolbox for grazing-incidence X-ray scattering data visualization and reduction, and indexing of buried three-dimensional periodic nanostructured films. J. Appl. Crystallogr. 48, 917–926 (2015).
Pettersson, H., Nik, S., Weidow, J. & Olsson, E. A method for producing site-specific TEM specimens from low contrast materials with nanometer precision. Microsc. Microanal. 19, 73–78 (2013).
Frisch, M. J. et al. Gaussian 16, Revision B.01 (Gaussian, Inc., 2016).
Acknowledgements
We thank C. Musumeci (Linköping University) for assistance with atomic force microscopy and S. Gustafsson (Chalmers) for assistance with TEM specimen preparation. This work was supported by the Knut and Alice Wallenberg foundation, VINNOVA (grant no. 2015-04859), the Swedish Research Council (grant agreement nos. 2016-03979, 2016-06146, 2016-05498, 2016-05990, 2018-03824), the Swedish Government Strategic Research Area in Materials Science on Functional Materials at Linköping University (Faculty Grant SFO Mat LiU no. 2009 00971), ÅForsk (18-313) and the European Research Council (ERC) under grant agreement no. 637624. We also thank the Chalmers Material Analysis Laboratory for their support of microscopes. T.P.R acknowledges funding from the Finnish Cultural Foundation and the Finnish Foundation for Technology Promotion. N.S. thanks the National Natural Science Foundation of China (grant no. 61805211). H.Y. acknowledges JST ALCA (JPMJAL1404) and the Futaba Foundation. Work at the University of Washington was supported by the National Science Foundation (DMR-1708450). D.F. acknowledges the Deutsche Forschungsgemeinschaft (DFG) for the grant ‘Molecular Understanding of Thermo-Electric Properties in Organic Polymers (FA 1502/1-1)’, and the Regional Computing Centre (RRZK) of Universität zu Köln, for providing computing time and resources on the HPC CHEOPS.
Author information
Authors and Affiliations
Contributions
S.F. conceived, designed and supervised the project. H.S. initiated the study. K.X. and H.S. prepared the samples, performed the electrical measurements and analysed the data. T.P.R. recorded and analysed the UV–vis–NIR data. G.W. performed the grazing-incidence wide-angle X-ray scattering and atomic force microscopy measurements. R.K. synthesized P(g42T-T) and P(g42T-TT). N.B.K. synthesized BBL, under S.A.J.’s supervision. Y.P. and W.M.C. performed and analysed the EPR data. X.L. and M.F. recorded and analysed the UPS spectra. D.F. performed the DFT calculations. K.S. and H.Y. performed and analysed the LEIPS data. C.Y.Y fabricated and tested the paper circuits. N.S. fabricated and tested the OLEDs. G.P., A.B.Y. and E.O. performed and analysed TEM. M.K. performed the kMC simulations. K.X., H.S., M.K., C.M. and S.F. wrote the manuscript. All authors contributed to discussion and manuscript preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–26, Table 1 and first principles calculations data.
Source data
Source Data Fig. 2
Source Data for electrical characterization of bilayers.
Source Data Fig. 3
Statistical Source Data for kinetic Monte Carlo simulation.
Source Data Fig. 4
Source Data for EPR and UV–vis–NIR spectroscopies.
Source Data Fig. 5
Source Data for electrical characterization of blend films.
Rights and permissions
About this article
Cite this article
Xu, K., Sun, H., Ruoko, TP. et al. Ground-state electron transfer in all-polymer donor–acceptor heterojunctions. Nat. Mater. 19, 738–744 (2020). https://doi.org/10.1038/s41563-020-0618-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-020-0618-7
This article is cited by
-
Ion-tunable antiambipolarity in mixed ion–electron conducting polymers enables biorealistic organic electrochemical neurons
Nature Materials (2023)
-
Ground-state electron transfer in all-polymer donor:acceptor blends enables aqueous processing of water-insoluble conjugated polymers
Nature Communications (2023)
-
Approaching disorder-tolerant semiconducting polymers
Nature Communications (2021)
-
A high-conductivity n-type polymeric ink for printed electronics
Nature Communications (2021)
-
Insights into the Ground-State Charge Transfer in Conjugated Polymer Donor–Acceptor Complexes
Journal of Electronic Materials (2021)