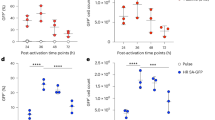
Abstract
Therapeutic T cells with desired specificity can be engineered by introducing T-cell receptors (TCRs) specific for antigens of interest, such as those from pathogens or tumour cells. However, TCR engineering is challenging, owing to the complex heterodimeric structure of the receptor and to competition and mispairing between endogenous and transgenic receptors. Additionally, conventional TCR insertion disrupts the regulation of TCR dynamics, with consequences for T-cell function. Here, we report the outcomes and validation, using five different TCRs, of the use of clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein 9 (Cas9) with non-virally delivered template DNA for the elimination of endogenous TCR chains and for the orthotopic placement of TCRs in human T cells. We show that, whereas the editing of a single receptor chain results in chain mispairing, simultaneous editing of α- and β-chains combined with orthotopic TCR placement leads to accurate αβ-pairing and results in TCR regulation similar to that of physiological T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data generated or analysed for this study are available within the paper and its Supplementary Information. The sequences of HDR DNA templates are provided in the Supplementary Dataset. Additional raw data are available from the corresponding author upon reasonable request.
References
June, C. H., Riddell, S. R. & Schumacher, T. N. Adoptive cellular therapy: a race to the finish line. Sci. Transl. Med. 7, 280ps7 (2015).
Kolb, H. J. et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 76, 2462–2465 (1990).
Rosenberg, S. A., Spiess, P. & Lafreniere, R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 233, 1318–1321 (1986).
Riddell, S. R. et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science 257, 238–241 (1992).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Price, D. A. et al. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21, 793–803 (2004).
Levine, A. G., Arvey, A., Jin, W. & Rudensky, A. Y. Continuous requirement for the TCR in regulatory T cell function. Nat. Immunol. 15, 1070–1078 (2014).
Cobbold, M. et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J. Exp. Med. 202, 379–386 (2005).
Neuenhahn, M. et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia 31, 2161–2171 (2017).
Blyth, E., Withers, B., Clancy, L. & Gottlieb, D. CMV-specific immune reconstitution following allogeneic stem cell transplantation. Virulence 7, 967–980 (2016).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Schrum, A. G., Turka, L. A. & Palmer, E. Surface T-cell antigen receptor expression and availability for long-term antigenic signaling. Immunol. Rev. 196, 7–24 (2003).
Gallegos, A. M. et al. Control of T cell antigen reactivity via programmed TCR downregulation. Nat. Immunol. 17, 379–386 (2016).
Dembić, Z. et al. Transfer of specificity by murine α and β T-cell receptor genes. Nature 320, 232–238 (1986).
Thomas, S. et al. Targeting the Wilms tumor antigen 1 by TCR gene transfer: TCR variants improve tetramer binding but not the function of gene modified human T cells. J. Immunol. 179, 5803–5810 (2007).
Bendle, G. M. et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat. Med. 16, 565–570 (2010).
van Loenen, M. M. et al. Mixed T cell receptor dimers harbor potentially harmful neoreactivity. Proc. Natl Acad. Sci. USA 107, 10972–10977 (2010).
Stanislawski, T. et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat. Immunol. 2, 962–970 (2001).
Cohen, C. J., Zhao, Y., Zheng, Z., Rosenberg, S. A. & Morgan, R. A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886 (2006).
Kuball, J. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2007).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–3903 (2007).
Scholten, K. B. J. et al. Codon modification of T cell receptors allows enhanced functional expression in transgenic human T cells. Clin. Immunol. 119, 135–145 (2006).
Voss, R.-H. et al. Coexpression of the T-cell receptor constant α domain triggers tumor reactivity of single-chain TCR-transduced human T cells. Blood 115, 5154–5163 (2010).
Bethune, M. T. et al. Domain-swapped T cell receptors improve the safety of TCR gene therapy. eLife 5, 1–24 (2016).
Govers, C. et al. TCRs genetically linked to CD28 and CD3ε do not mispair with endogenous TCR chains and mediate enhanced T cell persistence and anti-melanoma activity. J. Immunol. 193, 5315–5326 (2014).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nat. Med. 18, 807–815 (2012).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Mali, P., Esvelt, K. M. & Church, G. M. Cas9 as a versatile tool for engineering biology. Nat. Methods 10, 957–963 (2013).
Ren, J. et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 Inhibition. Clin. Cancer Res. 23, 2255–2266 (2017).
Legut, M., Dolton, G., Mian, A. A., Ottmann, O. & Sewell, A. CRISPR-mediated TCR replacement generates superior anticancer transgenic T-cells. Blood 131, 311–322 (2018).
Eyquem, J. et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 543, 113–117 (2017).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Schumann, K. et al. Generation of knock-in primary human T cells using Cas9 ribonucleoproteins. Proc. Natl Acad. Sci. USA 112, 10437–10442 (2015).
Suzuki, K. et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 540, 144–149 (2016).
Slaymaker, I. M. et al. Rationally engineered Cas9 nucleases with improved specificity. Science 351, 84–88 (2015).
Vakulskas, C. A. et al. A high-fidelity Cas9 mutant delivered as a ribonucleoprotein complex enables efficient gene editing in human hematopoietic stem and progenitor cells. Nat. Med. 24, 1216–1224 (2018).
Sommermeyer, D. et al. Designer T cells by T cell receptor replacement. Eur. J. Immunol. 36, 3052–3059 (2006).
Mastaglio, S. et al. NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood 130, 606–618 (2017).
Heemskerk, M. H. M. et al. Efficiency of T-cell receptor expression in dual-specific T cells is controlled by the intrinsic qualities of the TCR chains within the TCR–CD3 complex. Blood 109, 235–243 (2007).
Sommermeyer, D. & Uckert, W. Minimal amino acid exchange in human TCR constant regions fosters improved function of TCR gene-modified T cells. J. Immunol. 184, 6223–6231 (2010).
de Vree, P. J. P. et al. Targeted sequencing by proximity ligation for comprehensive variant detection and local haplotyping. Nat. Biotechnol. 32, 1019–1025 (2014).
Van Loenen, M. M. et al. Rapid re-expression of retrovirally introduced versusendogenous TCRs in engineered T cells afterantigen-specific stimulation. J. Immunother. 34, 165–174 (2011).
Knabel, M. et al. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat. Med. 8, 631–637 (2002).
Stemberger, C. et al. Lowest numbers of primary CD8+ T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood 124, 628–637 (2014).
Graef, P. et al. Serial transfer of single-cell-derived immunocompetence reveals stemness of CD8+ central memory T cells. Immunity 41, 116–126 (2014).
Riesenberg, S. & Maricic, T. Targeting repair pathways with small molecules increases precise genome editing in pluripotent stem cells. Nat. Commun. 9, 2164 (2018).
Dössinger, G. et al. MHC multimer-guided and cell culture-independent isolation of functional T cell receptors from single cells facilitates TCR identification for immunotherapy. PLoS ONE 8, e61384 (2013).
Nakagawa, S., Niimura, Y., Gojobori, T., Tanaka, H. & Miura, K. Diversity of preferred nucleotide sequences around the translation initiation codon in eukaryote genomes. Nucleic Acids Res. 36, 861–871 (2008).
Busch, D. H. & Pamer, E. G. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J. Immunol. 160, 4441–4448 (1998).
Acknowledgements
We thank members of the Busch laboratory for experimental help and critical discussion, particularly M. Hammel, F. Mohr, S. Dötsch, E. d’Ippolito and V. R. Buchholz. We also thank L. Germeroth (Juno Therapeutics) for critical discussion. This work was mainly supported by the German Centre for Infection Research (DZIF).
Author information
Authors and Affiliations
Contributions
K.Schober and D.H.B. conceived the study. K.Schober, T.R.M. and D.H.B. designed and analysed experiments. K.Schober, T.R.M. and F.G. performed CRISPR editing and transductions. F.G. performed double-stranded DNA production and gDNA analysis. S.G. performed FACS. T.R.M. and M.E. identified TCRs. M.E. produced pMHC reagents. K.Schober and T.R.M. performed flow cytometric analyses and functional assays. M.P., C.S., K.Schumann and A.M. advised on CRISPR–Cas9 RNP editing of T cells. T.L.R. and A.M. developed and advised on non-viral CRISPR–Cas9 large gene KI via HDR. K.Schober, T.R.M. and T.L.R. designed HDR DNA templates. K.Schober, T.R.M. and D.H.B. wrote the manuscript. All authors read and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.H.B. is co-founder of STAGE Cell Therapeutics GmbH (now Juno Therapeutics/Celgene) and T Cell Factory B.V. (now Kite/Gilead). D.H.B. has a consulting contract with and receives sponsored research support from Juno Therapeutics/Celgene. M.P. and C.S. are employees of Juno Therapeutics/Celgene. A.M. is on the scientific advisory board of PACT Pharma, serves as an advisor to Sonoma Biotherapeutics and previously served as an advisor to Juno Therapeutics and is a co-founder of Spotlight Therapeutics and Arsenal Biosciences. T.L.R. is a co-founder of Arsenal Biosciences. The Marson lab has received sponsored research support from Epinomics, Juno Therapeutics and Sanofi, and a gift from Gilead. A.M. and T.L.R. have previously filed related patent applications.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures.
Supplementary Dataset
Sequences of HDR DNA templates.
Rights and permissions
About this article
Cite this article
Schober, K., Müller, T.R., Gökmen, F. et al. Orthotopic replacement of T-cell receptor α- and β-chains with preservation of near-physiological T-cell function. Nat Biomed Eng 3, 974–984 (2019). https://doi.org/10.1038/s41551-019-0409-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-019-0409-0
This article is cited by
-
Unconventional human CD61 pairing with CD103 promotes TCR signaling and antigen-specific T cell cytotoxicity
Nature Immunology (2024)
-
Germline T cell receptor exchange results in physiological T cell development and function
Nature Communications (2023)
-
A gentler yield of ex vivo-edited T cells
Nature Biomedical Engineering (2023)
-
T cell receptor therapeutics: immunological targeting of the intracellular cancer proteome
Nature Reviews Drug Discovery (2023)
-
Cas9-induced targeted integration of large DNA payloads in primary human T cells via homology-mediated end-joining DNA repair
Nature Biomedical Engineering (2023)