Abstract
Background
Reduced prenatal growth followed by rapid postnatal weight gain are risk factors for developing metabolic and cardiovascular disease. Children reared in institutions experience a similar pattern of growth restriction followed by catch-up growth after removal. We explored whether patterns of catch-up growth affect metabolic and cardiovascular outcomes in previously institutionalized adolescents.
Method
A longitudinal study of institutionalized infants randomized to care as usual (n = 68) or foster care intervention (n = 68), and never institutionalized controls (n = 127). Body mass index (BMI) was measured at baseline (20 months), 30, 42 months, and ages 8, 12, 16. At age 16, metabolic and pro-inflammatory markers were derived from blood samples.
Results
Four BMI trajectories were derived (i.e., average-stable, low-stable, elevated, and accelerated). The accelerated trajectory was comprised predominately of children randomized to foster care, who also exhibited higher levels of glycosylated hemoglobin and C-reactive protein than the other three trajectories. Also, children placed in foster care at younger ages were more likely to be on the accelerated rather than the average-stable trajectory.
Conclusions
Although catch-up growth is viewed as a positive improvement among post-institutionalized children, rapid/continuous increases in body size pose a health concern. Attention should be given to monitoring weight gain, diet, and physical activity.
Similar content being viewed by others
Introduction
Young children reared in institutions often experience poor quality of care and low levels of cognitive and linguistic stimulation; in some institutions, children also may be malnourished. This confluence of nutritional and psychosocial deprivation often results in growth restriction.1 At the time of adoption, children reared in institutions across the world are smaller for their age, but exhibit dramatic gains in height and weight after adoption into nurturing environments.2 Catch-up growth is observed in the few longer-term longitudinal studies with assessments into middle childhood to early adolescence, as height and weight of most adoptees are comparable to standardized means of growth charts and/or typical children.1,3,4,5 Although considered a positive improvement for adoptees, rapid catch-up growth can contribute to risk for overweight/obesity and related health problems in later adolescence and adulthood.6,7,8
Rapid catch-up growth, often observed in babies born at low birth weight and growth-stunted children who experience significant weight gain, is a risk for obesity, type two diabetes, hypertension, and coronary heart disease in adulthood.6,7,8,9 Studies of the effects of famine10,11,12 and experiments using animal models13,14 examining the effects of shifting from perinatal under-nutrition to adequate/rich nutrition environments suggest that rapid, rather than gradual, catch-up growth is associated with risk for overweight or obesity and having more centralized body fat. Although prenatal factors, such as low birth weight and being small for gestational age, are risks for developing cardiometabolic problems, a radical change in body size during the postnatal period is always evident in the disease process even among typically developing children.8,15,16,17 Also, rapid weight gain in infancy rather than later developmental periods may have detrimental effects.8,15,16,17,18,19
Previous work by our group has shown that Romanian children with a history of institutionalization randomized to foster care (FCG) show significant catch-up growth compared to controls who received care as usual (CAUG),1 consistent with longitudinal observations in post-institutionalized youth.3,4,5 However, no studies have examined relations between patterns of catch-up growth and metabolic and inflammatory biomarkers in late adolescence among post-institutionalized adolescents. Of note, inflammation plays a role in the pathogenesis of obesity and diabetes, which have been linked to elevated levels in a cluster of low-grade pro-inflammatory cytokines, including interleukin (IL)- 6, tumor necrosis factor (TNF)- α, and c-reactive protein (CRP; an acute phase protein released in the liver in response to systemic inflammation).20,21 Elevated levels of CRP, IL-6, and TNF- α have been associated with greater risk for cardiovascular disease21 and IL-8 is related to atherosclerosis, respectively.20 Glycosylated hemoglobin (HbA1c), an index used to monitor the average blood glucose in the past 2–3 months among individuals with diabetes (American Diabetes Association, VA) is also elevated among individuals who are overweight. Given that overweight and obese youth often become overweight or obese adults, which in turn elevates their risk for metabolic and cardiovascular disease,22 identifying growth patterns that are associated with metabolic risk and inflammation is important.
In this study, we examined patterns of catch-up growth and their associations with metabolic and inflammatory markers among previously institutionalized Romanian adolescents in a randomized controlled trial of foster care as an alternative of institutional care (Bucharest Early Intervention Project [BEIP]). We had two goals. The first goal was to examine whether BMI trajectories across 16 years and BMI at age 16 differed among post-institutionalized adolescents (FCG, CAUG), and a demographically-matched never institutionalized group (NIG). The second goal was to use an exploratory analysis to identify individuals exhibiting distinct BMI trajectories and to determine their associations with placement and timing of foster care intervention, and metabolic (HbA1c) and pro-inflammatory (CRP, IL- 6,-8, and TNF- α) outcomes at age 16.
Method
Participants
Trial design and participant selection have been previously reported23 and are summarized in Fig. 1. Physical examination was completed on 187 infants ranging from 6 to 31 months who were living in one of six institutions in Bucharest, Romania; 51 children were excluded for serious medical conditions (e.g., genetic and fetal alcohol syndromes). Accordingly, 136 children (ages 6–30 months) were recruited. After the baseline assessment, half of the children were randomly assigned to care as usual (CAUG: n = 68) or to foster care (FCG: n = 68).23 At baseline, a group of age- and gender-matched never institutionalized children (NIG: n = 72) was recruited from pediatric clinics in Bucharest; additional NIG were recruited at age 8 (n = 61).
Participation varied across data collection. The trajectory analyses in this report included 261 participants with at least one data point of BMI (68 CAUG, 35 female; 66 FCG, 34 female; 127 NIG, 69 female). At age 16, a subgroup of the participants (n = 127: 44 CAUG, 41 FCG, 42 NIG) from our larger study provided dried blood spots, which were used to derive metabolic and inflammatory biomarkers (participant characteristics in Supplemental Table S1). This subgroup is representative of individuals who provided data at age 16 (range = 15.49–17.97 years), were available, and agreed to participate in the bloodspot assessment (13 participants declined). Among the FCG and CAUG who provided blood samples at age 16 versus those who did not, there were no differences in sex (p = 0.97), ethnicity (p = 0.08), birth weight (p = 0.49), baseline BMI (p = 0.32), and baseline age (p = 0.56).
Study procedures were approved by local commissions on child protection in Bucharest, the Romanian ministry of health, an ethics committee of Bucharest University, and institutional review boards of the institutions of the three principal investigators. Consent was obtained from children’s legal guardian and assent was obtained from the children at age 8, 12, and 16 for each procedure.
Measures of body size
Height and weight were measured at baseline (M age = 20.44 months; SD = 7.30), 30 months (M age = 30.73 months; SD = 0.88), 42 months (M age = 42.53 months; SD = 0.64), 8 years (M age = 102.00 months; SD = 4.86), 12 years (M age = 151.73 months; SD = 6.37), and 16 years (M age = 201.07 months; SD = 7.05). Height and weight were used to calculate BMI, which was converted into age- and sex-standardized z-scores using the World Health Organization international growth references.24 BMI z-scores were used in trajectory analyses as it adjusts for population regression to the mean, so differences reflect catch-up growth. At age 16, BMI z-scores ≥ 1 is defined as overweight.24
Metabolic and pro-inflammatory outcomes at age 16
Participants provided dried blood spots (DBS), a minimally invasive technique to measure blood-based biomarkers. Trained research assistants cleaned participants’ finger with isopropyl alcohol then pricked with a sterile, disposable, micro-lancet. The first drop of blood was wiped away. The next drop of blood was collected with a microcuvette and immediately analyzed using a point of care device to measure glycosylated hemoglobin A1c (HbA1c) (HemoCue® 501 analyzer; 4 μL of blood).25 HbA1c is reported as a percentage of total hemoglobin and represents the average blood glucose levels over the past 2–3 months.
Four subsequent blood drops (each ~50 μL) were applied to filter paper. The blood drops saturated the paper and was air-dried for at least 4 h. After drying, DBS samples were placed into a re-sealable plastic bag and stored at −24 °C until they were shipped to the Laboratory for Human Biology Research (Evanston, IL) for processing. Four pro-inflammatory cytokines were derived. C-reactive protein (CRP) was measured using a high-sensitivity CRP assay method;26 interleukin (IL)-6 was measured using a modified Bloodspot protocol of the R&D Systems Quantikine HS Human IL-6 Kit# HS600B; IL-8 and tumor necrosis factor-α (TNF-α) were assayed using a modified Bloodspot protocol of the Meso Scale Discovery V-PLEX Custom Human Cytokine kit# K151A0H-1. Between-assay coefficients of variability for low, mid, and high samples were 12.35, 4.23, 6.98 pg/ml for CRP, 12.75, 6.97, 4.77 pg/ml for IL-6, 9.69, 3.32, 8.19 pg/ml for IL-8, 37.00, 14.13, 6.34 pg/ml for TNF-α. There were 14 individuals with CRP assay results below the limit of detection ( < 0 mg/L); these values were winsorized with the nearest value within the limit of detection. No CRP values exceeded 10 mg/L.
Covariates
At the DBS collection, participants’ body temperature was measured twice with an infrared ear thermometer. The average body temperature, sex, and use of any medication (yes/no) were used as covariates in analyses involving pro-inflammatory outcomes. For HbA1c, two participants taking drugs to control glucose levels were excluded. Additionally, one pregnant and one breast-feeding female were excluded from all analyses involving metabolic and pro-inflammatory outcomes.
Data analyses
BMI trajectories
Variable-centered trajectories of BMI z-scores were estimated using growth curve analysis in SPSS27 to provide fixed group trends and differences in the mean rate of change based on a variable consisting of pre-defined groups. In this procedure, repeated measures of BMI were regressed on the timing of assessments (i.e., age from baseline to age 16) to estimate variability at baseline (i.e., intercept variance) and rates of change for individuals (i.e., slope variance). First, unconditional models including a random intercept and fixed slope testing polynomial functions of age (i.e., age, age², and age³) were performed across the entire sample to identify the optimal growth function of BMI. The -2 log likelihood was compared across the polynomial models and the quadratic model showed the best fit (-2 log likelihood for quadratic: 2577.66 vs 2596.45 for linear and 2576.45 for cubic; the cubic term was not statistically significant). Second, we expanded on the best fitting unconditional model by including age by group (CAUG = reference, FCG, NIG) interactions to examine whether foster care moderated growth patterns among institutionalized children. To contrast FCG from typical development, NIG was coded as the reference. Full information maximum likelihood estimation (FIML) was used to handle missing data and obtain unbiased estimates. Age was centered on the first time point so the intercept represents estimates of BMI z-score at baseline; to obtain estimates of BMI at age 16, age was centered on the last time point.
Person-centered BMI trajectories were derived with Latent Class Growth Analysis (LCGA) in Mplus, version 7. Unlike the previous variable-centered approach that assumes individuals in the same group are on the same trajectory, LCGA is an exploratory technique that identifies subgroups of individuals within a larger heterogeneous population based on individual response patterns over time. Following recommended steps, we conducted LCGA with a quadratic function and estimated two to five classes using FIML. Successive comparisons of model fit indices, including Bayesian Information Criterion, likelihood ratio tests, and entropy values, suggested the best model fit. All models and percentage of participants in each trajectory were inspected. Age was centered on the first time point.
To assess whether person-centered BMI trajectories differed in metabolic (HbA1c) and pro-inflammatory (CRP, IL-6, IL-8, TNF-α) biomarkers, while adjusting for covariates, we performed non-parametric approximate general independence permutation tests based on 10000 Monte-Carlo resamplings in the R package “coin”.28 Non-parametric permutation tests are optimal for hypothesis testing in smaller samples and do not rely on a theoretic distribution or normality of data. Pairwise permutation tests were performed when the initial one-way test was statistically significant. Complementary bootstrapping analyses that are more suitable for large sample sizes showed similar results (Supplemental Figure S1).
Results
Variable-centered trajectories
Figure 2a displays the BMI trajectories by group. As seen in Table 1, FCG and CAUG had comparable BMI at baseline. Whereas the FCG exhibited a relatively rapid pattern of increase in BMI that decelerated by adolescence, the CAUG showed a relatively gradual pattern. Compared to NIG, FCG showed lower BMI at baseline and no difference in rates of change. At age 16, BMI was comparable across the three groups and within the normal range.
Person-centered trajectories
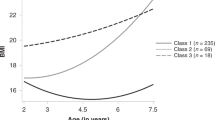
Fit indices from LCGA suggested that a 4-class model provided the best fit (Supplemental Table S2). Four trajectories were identified (Fig. 2b), including an average-stable BMI trajectory consisting of about half the sample (46.5%, n = 136), a low-stable trajectory (17.6%, n = 41), an elevated trajectory (20.3%, n = 56), and an accelerated trajectory (15.6%, n = 28). As seen in Table 2a, the average-stable and low-stable trajectories showed no change in BMI z-scores across age, though the low-stable trajectory began with a lower BMI z-score at baseline. Whereas the elevated trajectory began with a higher BMI z-score, the accelerated trajectory began with a lower BMI z-score; however, both trajectories showed significant increases in BMI z-scores that decelerated by adolescence and both were overweight at age 16 (i.e., BMI z-scores ≥ 1).24
Half of the accelerated trajectory (50%) consisted of FCG; the majority of the elevated (66.1%) and average-stable (50.7%) trajectories were NIG (Table 2b). Though table 2B shows column percentages for trajectory class membership by study group, row percentages are in Supplemental Table S3. Trajectories were proportionately distributed across sex. Individuals in the accelerated and low-stable trajectories were born at earlier gestation age compared to the average-stable trajectory. The low-stable trajectory was also born at lower birth weight compared to the other three trajectories. These results are consistent with the fact that the accelerated and low-stable trajectories were largely consisted of FCG and CAUG who were born at lower birth weight than NIG.1
To examine timing effects of foster care placement on growth patterns, we performed a multinomial logistic regression model regressing the four BMI trajectories (with the average-stable normative trajectory as the reference) on age of foster care placement while accounting for birth weight and gestation age among the FCG. Mean age for foster care placement was 22.63 months (SD = 7.33; range = 6.81–33.01; n = 65). FCG placed in foster care later were less likely to be in the accelerated trajectory relative to the average-stable trajectory (OR = 0.87, 95% CI = 0.77, 0.98, p = 0.024). Timing of foster care placement were not related to odds of being in the low-stable (OR = 0.98, 95% CI = 0.80, 1.03, p = 0.14) or elevated (OR = 0.94, 95% CI = 0.83, 1.07, p = 0.37) trajectories relative to the average-stable trajectory.
Associations with metabolic and pro-inflammatory markers at age 16 adjusted for covariates
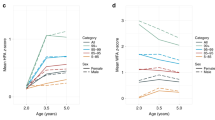
BMI trajectories differed on levels of HbA1c (p = 0.04) and CRP (p = 0.001). The accelerated trajectory showed significantly greater levels of HbA1c and CRP than the average-stable and low-stable trajectories, and greater levels at trend level compared to the elevated trajectory (Fig. 3). There were no differences in IL-6 (p = 0.75), IL-8 (p = 0.30), and TNF-α (p = 0.71).
Discussion
This prospective longitudinal study examined growth patterns across baseline (M age = 20 months) to age 16 and their associations with metabolic and inflammatory biomarkers among post-institutionalized Romanian adolescents and typically developing controls. Findings revealed four distinct trajectories of BMI z-scores, including average-stable, low-stable, elevated, and accelerated trajectories. Consistent with our hypotheses, an accelerated trajectory, reflective of rapid catch-up growth, was over-represented by the FCG who experienced restricted early growth and subsequent catch-up growth. This accelerated trajectory showed elevated levels of HbA1c and CRP at age 16 compared to the average-stable and low-stable trajectories, as well as the elevated trajectory (at trend level). Moreover, earlier age of placement into foster care during infancy among the FCG was linked to higher risk for being on an accelerated rather than the normative average-stable BMI trajectory. These findings suggest that different patterns of growth—for the ever-institutionalized adolescents, resulting from random assignment and timing of placement in foster care or to remain in institutions—may play a role in the development of overweight or obesity and related health risks by late adolescence.
Why is the FCG linked to a rapid catch-up growth risk profile?
The FCG and CAUG were born at lower birth weight and earlier gestation age and experienced growth restriction while living in institutions.1 These factors, while confounded in the current sample, all reflect impaired intrauterine and early postnatal growth. Impaired prenatal growth can alter the metabolic-endocrine systems, leading to insulin-resistance and failure of ß-cell compensation, which mediate later risk for metabolic and cardiovascular diseases when paired with rapid postnatal weight gain.29,30 Specific biological alterations are still under investigation, but include reduced pancreatic vascularization, insulin content and secretion, increased adiposity, and epigenetic changes in metabolic pathways extending into the postnatal period.31,32 As evident in the variable-centered analysis, the FCG showed a relatively rapid catch-up growth pattern, whereas CAUG showed a relatively gradual catch-up growth pattern. We speculate that the FCG and CAUG might have metabolic-endocrine changes in early life due to growth restriction and stress, but the FCG additionally shifted from a poor to enriched/ adequate nutrition environment, which might accompany further biological changes. However, not all the FCG exhibited this accelerated BMI risk profile.
The higher prevalence of accelerated growth in the FCG removed from institutions earlier versus the higher prevalence of average-stable growth in FCG removed later suggest that some biological changes are less likely if the shift to rapid weight gain occur past a certain age. This finding converges with animal models that identified the preweaning period as a sensitive window for rapid weight gain.13,14,33 Human studies also suggest rapid weight gain during the first two years of life or earlier (e.g., first 3 or 6 months) is strongly related to later obesity and metabolic changes across typical infants15,16,17 and infants born small for gestational age.17,18,19 Environmental insults or experiences during periods with high cell replication and differentiation can be damaging to the development and programming of certain tissue and organs.34 Here, we extended the associations among cardiometabolic risks, postnatal growth restriction, and the timing of rapid infant weight gain to a special population. Our findings suggest that similar biological changes in the metabolic-endocrine systems linked to shifting from early growth restriction to rapid weight gain in infancy may operate within our sample of post-institutionalized adolescents. However, we cannot rule out the relevance of later developmental periods.
The finding that the accelerated trajectory showed higher HbA1c and CRP than the average-stable and low-stable trajectories, but not the elevated trajectory, is consistent with reports showing greater risk of cardiovascular and metabolic disease among obese/overweight individuals across childhood and adulthood.22 The lack of statistical difference between the accelerated and elevated trajectories could be due to inadequate power or could suggest that different developmental pathways characterized by either continuous increases in weight gain or stable overweight may lead to the same risks. The latter interpretation is consistent with a recent study that found BMI trajectories characterized by progressive increases in BMI or persisting overweight/obesity from ages 6 to 49 are at greater risk for cardiovascular disease, dyslipidemia, and hypertension, compared to a stable-average BMI trajectory.35 At the biological level, adiposity is strongly related to higher levels of TNF- α and IL-6 in adipose tissue; in turn, pro-inflammatory cytokines, predominately IL-6, drive the increases in CRP.20,21
However, we did not observe differences in IL-6,-8, and TNF-α across BMI trajectories, as we had expected. BMI is a rough measure of body fat, as opposed to measures of visceral fat and fat mass, which would provide more robust associations with inflammatory processes. Previous studies examining immune biomarkers have shown associations particularly between elevated CRP levels and cardiovascular risk markers, including higher BMI and blood pressure in children and adolescents,36,37,38 and in adults with cardiovascular disease and diabetes.21 Also, prediabetes is often observed before developing type 2 diabetes and contributes to risk for cardiovascular disease. Given that overweight/obesity in adolescence likely continues into adulthood, individuals in accelerated and elevated BMI trajectories are projected to experience chronic metabolic and cardiovascular problems, such as type 2 diabetes, hypertension, and atherosclerosis, in later adulthood, compared to individuals in low-stable or average-stable BMI trajectories.
The implications of this study is that children should still be removed from institutions as early as possible. Catch-up in height enhances cognitive functioning (i.e., IQ) in our FCG sample1 and in children with restricted prenatal growth.39 Also, earlier foster care placement predicts positive outcomes across developmental domains, including secure attachment, less behavioral problems, and better cognitive and brain functioning.40 Also, poor growth has risks, such as susceptibility to common infectious diseases. The practical application of this research is that physicians and nutritionist should work with adoptive parents to devise plans for healthy catch-up growth that maximizes benefits and diminishes health risks. For example, informing adoptive parents about the possible consequences of shifting from perinatal restricted growth and rapid weight gain in infancy, planning a balanced diet with adequate nutrition and limiting obesogenic foods, planning regular physical activities, and starting these preventive measures as early as possible with their children.
Our results are interpreted with respect to several strengths and limitations. Our longitudinal analyses included a reasonably powered sample size and showed predictive validity for groups and outcomes. We also ensured reliability of our results from the trajectory analyses by performing the same models for individuals with at least two data points of BMI (i.e., those with repeated assessments); The results were the same as results obtained with individuals with at least one data point of BMI. However, the shape and number of trajectories are driven by the characteristics of our sample (e.g., institutionalization and foster care during infancy) and may not reflect other populations of children institutionalized or placed in homes at other stages of development. To maximize participation, we selected a less invasive finger-prick blood method, as opposed to venous blood draw. Although finger-prick blood has been validated as proxies of serum level and the lab that conducted our assays obtained patterns of variability that are comparable to serum assays, we note that there may be variability in finger-prick blood. Because the metabolic and inflammatory measures were collected from a smaller subsample, we used permutations tests to ensure that group differences did not appear by chance. This smaller sample may have limited power to detect differences between BMI trajectories (i.e., accelerated vs elevated trajectories). Similarly, the subgroup analyses involving timing of foster care placement within the FCG involves a relatively small sample, as such, our findings warrant caution in interpretation and replication. Also, we randomized the caregiving environment and not nutrition, though nutrition and care of FCG are presumed to be enhanced relative to CAUG. For example, infants in institutions were fed with less social interactions and with food lacking in variety. Future studies would benefit from larger samples, measures of tissue mass and important determinants of growth and health risks that we lacked, including the child’s genetic risk, maternal pregnancy history, birth status, caregiver influences (e.g., feeding behavior), and physical activity. To understand the relations among growth patterns, cardiometabolic, and neurocognitive outcomes in post-institutionalized children, future studies should examine the effects of catch-up growth in height or weight and cardiometabolic markers on brain functioning, performance on neuropsychological tests, and academic achievements.
In conclusion, early growth restriction followed by rapid catch-up growth is not directly related to an unfavorable cardiovascular and metabolic profile at age 16 among post-institutionalize adolescents. However, infants removed from institutions earlier were more likely to exhibit an accelerated growth profile linked to cardiometabolic risks.
References
Johnson, D. E. et al. Growth and associations between auxology, caregiving environment, and cognition in socially deprived Romanian children randomized to foster vs ongoing institutional care. Arch. Pedia. Adol Med. 164, 507–516 (2010).
Van IJzendoorn, M. H., Bakermans-Kranenburg, M. J. & Juffer, F. Plasticity of growth in height, weight, and head circumference: meta-analytic evidence of massive catch-up after international adoption. J. Dev. Behav. Pediatr. 28, 334–343 (2007).
Le Mare, L. & Audet, K. A longitudinal study of the physical growth and health of postinstitutionalized Romanian adoptees. Paediatr. Child Heal. 11, 85–91 (2006).
Reid, B. M. et al. Early growth faltering in post-institutionalized youth and later anthropometric and pubertal development. Pediatr. Res 82, 278–284 (2017).
Sonuga-Barke, E. J., Schlotz, W. & Rutter, M. Physical growth and maturation following early severe institutional deprivation: do they mediate specific psychopathological effects? Monogr. Soc. Res Child 75, 143–166 (2010).
Adair, L. & Cole, T. J. Rapid child growth raises blood pressure in adolescent boys who were thin at birth. Hypertension 41, 451–456 (2003).
Barker, D. J., Osmond, C., Forsén, T. J., Kajantie, E. & Eriksson, J. G. Trajectories of growth among children who have coronary events as adults. New Engl. J. Med. 353, 1802–1809 (2005).
Eriksson, J. G. et al. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 318, 427–431 (1999).
Popkin, B. M., Richards, M. K. & Montiero, C. A. Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J. Nutr. 126, 3009–3016 (1996).
Shi, Z. et al. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J. Hypertens. 36, 54–60 (2018).
Stein, A., Zybert, P., Van de Bor, M. & Lumey, L. Intrauterine famine exposure and body proportions at birth: the Dutch Hunger Winter. Int J. Epidemiol. 33, 831–836 (2004).
Stanner, S. & Yudkin, J. Fetal programming and the Leningrad Siege study. Twin Res Hum. Genet 4, 287–292 (2001).
Ozanne, S. E. & Hales, C. N. Catch-up growth and obesity in male mice. Nature 427, 411–412 (2004).
Shin, B. C., Dai, Y., Thamotharan, M., Gibson, L. C. & Devaskar, S. U. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J. Neurosci. Res 90, 1169–1182 (2012).
Ekelund, U. et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES). Am. J. Clin. Nutr. 83, 324–330 (2006).
Ong, K. K. & Loos, R. J. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 95, 904–908 (2006).
Baird, J. et al. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 331, 929 (2005).
Leunissen, R. W., Kerkhof, G. F., Stijnen, T. & Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 301, 2234–2242 (2009).
Singhal, A. et al. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am. J. Clin. Nutr. 92, 1133–1144 (2010).
Kim, C. S. et al. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J. Obes. 30, 1347–1355 (2006).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11, 98–107 (2011).
Simmonds, M., Llewellyn, A., Owen, C. G. & Woolacott, N. Predicting adult obesity from childhood obesity: a systematic review and meta-analysis. Obes. Rev. 17, 95–107 (2015).
Zeanah, C. H. et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Dev. Psychopathol. 15, 885–907 (2003).
de Onis, M. D. WHO child growth standards based on length/height, weight and age. Acta Paediatr. 95, 76–85 (2007).
Andersson, A., Lindh, J. & Eriksson, A. Evaluation of the HemoCue HbA1c 501 system in primary care settings. Point Care: J. Near Patient Test. Technol. 16, 128–130 (2017).
McDade, T. W. High-sensitivity enzyme immunoassay for C-reactive protein in dried blood spots. Clin. Chem. 50, 652–654 (2004).
Singer, J. D. & Willett, J. B. Applied Longitudinal Data Analysis. (Oxford University Press, New York, 2003).
Hothorn, T., Hornik, K., Wiel, M. A. & Zeileis, A. Implementing a class of permutation tests: ThecoinPackage. J. Stat. Softw. 28, 1–23 (2008).
Berends, L. M., Fernandez-Twinn, D. S., Martin-Gronert, M. S., Cripps, R. L. & Ozanne, S. E. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J. Pediatr. Obes. 37, 1051–1057 (2012).
Ong, K. K. et al. Insulin sensitivity and secretion in normal children related to size at birth, postnatal growth, and plasma insulin-like growth factor-I levels. Diabetologia 47, 1064–1070 (2004).
Warner, M. J. & Ozanne, S. E. Mechanisms involved in the developmental programming of adulthood disease. Biochem J. 427, 333–347 (2010).
Relton CL, Davey-Smith G, Ozanne SE. Developmental epigenetic programming in diabetes and obesity. In eds Jirtle R, Tyson E. Environmental Epigenomics in Health and Disease. Berlin: Springer; 2013: 235-253.
Lewis, D. S. et al. Preweaning food intake influences the adiposity of young adult baboons. J. Clin. Invest 78, 899–905 (1986).
Rueda-Clausen, C. F., Morton, J. S. & Davidge, S. T. The early origins of cardiovascular health and disease: who, when, and how. Sem Reprod Med. 29, 97–210 (2011).
Buscot, M. J. et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur. Heart J. 39, 2263–2270 (2018).
Ford, E. S. C-reactive protein concentration and cardiovascular disease risk factors in children: findings from the National Health and Nutrition Examination Survey 1999–2000. Circulation 108, 1053–1058 (2003).
Juonala, M. Childhood C-reactive protein in predicting CRP and carotid intima-media thickness in adulthood: The Cardiovascular Risk in Young Finns Study. Arter. Throm Vas. 26, 1883–1888 (2006).
Steene-Johannessen, J., Kolle, E., Reseland, J. E., Anderssen, S. A. & Andersen, L. B. Waist circumference is related to low-grade inflammation in youth. Int J. Pediatr. Obes. 5, 313–319 (2010).
Ong, K. K. et al. Postnatal growth in preterm infants and later health outcomes: a systematic review. Acta Paediatr. 104, 974–986 (2015).
Zeanah, C. H., Humphreys, K. L., Fox, N. A. & Nelson, C. A. Alternatives for abandoned children: insights from the Bucharest Early Intervention Project. Curr. Opin. Psychol. 15, 182–188 (2017).
Funding Source
The John D. and Catherine T. MacArthur Foundation, the Binder Family Foundation, the Palix Foundation, the Jacobs Foundation, and the National Institute of Mental Health (R01MH091363) to C.A.N. and the Palix Foundation to C.H.Z..
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tang, A., Slopen, N., Nelson, C.A. et al. Catch-up growth, metabolic, and cardiovascular risk in post-institutionalized Romanian adolescents. Pediatr Res 84, 842–848 (2018). https://doi.org/10.1038/s41390-018-0196-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-018-0196-4
This article is cited by
-
The association of gestational age and birthweight with blood pressure, cardiac structure, and function in 4 years old: a prospective birth cohort study
BMC Medicine (2023)
-
Catch-up growth in juvenile rats, fat expansion, and dysregulation of visceral adipose tissue
Pediatric Research (2022)
-
Liver transcriptome profiling and functional analysis of intrauterine growth restriction (IUGR) piglets reveals a genetic correction and sexual-dimorphic gene expression during postnatal development
BMC Genomics (2020)
-
Identifying typical trajectories in longitudinal data: modelling strategies and interpretations
European Journal of Epidemiology (2020)