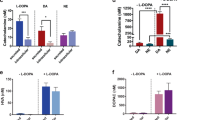
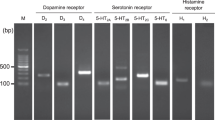
Abstract
Although long-studied in the central nervous system, there is increasing evidence that dopamine (DA) has important roles in the periphery including in metabolic regulation. Insulin-secreting pancreatic β-cells express the machinery for DA synthesis and catabolism, as well as all five DA receptors. In these cells, DA functions as a negative regulator of glucose-stimulated insulin secretion (GSIS), which is mediated by DA D2-like receptors including D2 (D2R) and D3 (D3R) receptors. However, the fundamental mechanisms of DA synthesis, storage, release, and signaling in pancreatic β-cells and their functional relevance in vivo remain poorly understood. Here, we assessed the roles of the DA precursor l-DOPA in β-cell DA synthesis and release in conjunction with the signaling mechanisms underlying DA’s inhibition of GSIS. Our results show that the uptake of l-DOPA is essential for establishing intracellular DA stores in β-cells. Glucose stimulation significantly enhances l-DOPA uptake, leading to increased DA release and GSIS reduction in an autocrine/paracrine manner. Furthermore, D2R and D3R act in combination to mediate dopaminergic inhibition of GSIS. Transgenic knockout mice in which β-cell D2R or D3R expression is eliminated exhibit diminished DA secretion during glucose stimulation, suggesting a new mechanism where D2-like receptors modify DA release to modulate GSIS. Lastly, β-cell-selective D2R knockout mice exhibit marked postprandial hyperinsulinemia in vivo. These results reveal that peripheral D2R and D3R receptors play important roles in metabolism through their inhibitory effects on GSIS. This opens the possibility that blockade of peripheral D2-like receptors by drugs including antipsychotic medications may significantly contribute to the metabolic disturbances observed clinically.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Fleischhacker WW, Siu CO, Boden R, Pappadopulos E, Karayal ON, Kahn RS et al. Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European First-Episode Schizophrenia Trial. Int J Neuropsychopharmacol. 2013;16:987–95.
Fabrazzo M, Monteleone P, Prisco V, Perris F, Catapano F, Tortorella A, et al. Olanzapine is faster than haloperidol in inducing metabolic abnormalities in schizophrenic and bipolar patients. Neuropsychobiology. 2015;72:29–36.
Freyberg Z, Aslanoglou D, Shah R, Ballon JS. Intrinsic and antipsychotic drug-induced metabolic dysfunction in schizophrenia. Front Neurosci. 2017;11:432.
Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Borglum AD, et al. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. Am J Psychiatry. 2017;174:686–94.
Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, et al. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–90.
Ballon JS, Pajvani U, Freyberg Z, Leibel RL, Lieberman JA. Molecular pathophysiology of metabolic effects of antipsychotic medications. Trends Endocrinol Metab. 2014;25:593–600.
Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20.
Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73:317–32.
Suzuki M, Hurd YL, Sokoloff P, Schwartz JC, Sedvall G. D3 dopamine receptor mRNA is widely expressed in the human brain. Brain Res. 1998;779:58–74.
Rubi B, Ljubicic S, Pournourmohammadi S, Carobbio S, Armanet M, Bartley C, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–32.
Simpson N, Maffei A, Freeby M, Burroughs S, Freyberg Z, Javitch J, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26:1757–72.
Ustione A, Piston DW. Dopamine synthesis and D3 receptor activation in pancreatic beta-cells regulates insulin secretion and intracellular [Ca(2 + )] oscillations. Mol Endocrinol. 2012;26:1928–40.
Garcia-Tornadu I, Ornstein AM, Chamson-Reig A, Wheeler MB, Hill DJ, Arany E, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151:1441–50.
Rosati G, Maioli M, Aiello I, Farris A, Agnetti V. Effects of long-term L-dopa therapy on carbohydrate metabolism in patients with Parkinson’s disease. Eur Neurol. 1976;14:229–39.
Ericson LE, Hakanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic B-cells following injection of L-DOPA. Localization to secretory granules and inhibition of insulin secretion. Diabetologia. 1977;13:117–24.
Zern RT, Bird JL, Feldman JM. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster. Diabetologia. 1980;18:341–6.
Ustione A, Piston DW, Harris PE. Minireview: Dopaminergic regulation of insulin secretion from the pancreatic islet. Mol Endocrinol. 2013;27:1198–207.
Moritz AE, Free RB, Sibley DR. Advances and challenges in the search for D2 and D3 dopamine receptor-selective compounds. Cell Signal. 2018;41:75–81.
Diaz-Torga G, Feierstein C, Libertun C, Gelman D, Kelly MA, Low MJ, et al. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology. 2002;143:1270–9.
Kelly MA, Rubinstein M, Asa SL, Zhang G, Saez C, Bunzow JR, et al. Pituitary lactotroph hyperplasia and chronic hyperprolactinemia in dopamine D2 receptor-deficient mice. Neuron. 1997;19:103–13.
Wang GJ, Volkow ND, Fowler JS. The role of dopamine in motivation for food in humans: implications for obesity. Expert Opin Ther Targets. 2002;6:601–9.
Garcia-Tornadu I, Perez-Millan MI, Recouvreux V, Ramirez MC, Luque G, Risso GS, et al. New insights into the endocrine and metabolic roles of dopamine D2 receptors gained from the Drd2 mouse. Neuroendocrinology. 2010;92:207–14.
Iturriza FC, Thibault J. Immunohistochemical investigation of tyrosine-hydroxylase in the islets of Langerhans of adult mice, rats and guinea pigs. Neuroendocrinology. 1993;57:476–80.
Persson-Sjogren S, Forsgren S, Taljedal IB. Tyrosine hydroxylase in mouse pancreatic islet cells, in situ and after syngeneic transplantation to kidney. Histol Histopathol. 2002;17:113–21.
Takayanagi M, Watanabe T. Immunocytochemical colocalizations of insulin, aromatic L-amino acid decarboxylase, dopamine beta-hydroxylase, S-100 protein and chromogranin A in B-cells of the chicken endocrine pancreas. Tissue Cell. 1996;28:17–24.
Ganic E, Johansson JK, Bennet H, Fex M, Artner I. Islet-specific monoamine oxidase A and B expression depends on MafA transcriptional activity and is compromised in type 2 diabetes. Biochem Biophys Res Commun. 2015;468:629–35.
Maffei A, Marie Segal A, Alvarez-Perez JC, Garcia-Ocana A, Harris P. Anti-incretin, anti-proliferative action of dopamine on beta-cells. Mol Endocrinol. 2015;29:542–57.
Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–78.
Romanova IV, Derkach KV, Mikhrina AL, Sukhov IB, Mikhailova EV, Shpakov AO. The leptin, dopamine and serotonin receptors inhypothalamic POMC-neurons of normal and obese rodents. Neurochem Res. 2018;43:821–837.
Newman AH, Grundt P, Cyriac G, Deschamps JR, Taylor M, Kumar R, et al. N-(4-(4-(2,3-dichloro- or 2-methoxyphenyl)piperazin-1-yl)butyl)heterobiarylcarboxamides with functionalized linking chains as high affinity and enantioselective D3 receptor antagonists. J Med Chem. 2009;52:2559–70.
Xiao J, Free RB, Barnaeva E, Conroy JL, Doyle T, Miller B, et al. Discovery, optimization, and characterization of novel D2 dopamine receptor selective antagonists. J Med Chem. 2014;57:3450–63.
Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–22.
Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, et al. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci. 2011;14:1033–8.
Jung MY, Skryabin BV, Arai M, Abbondanzo S, Fu D, Brosius J, et al. Potentiation of the D2 mutant motor phenotype in mice lacking dopamine D2 and D3 receptors. Neuroscience. 1999;91:911–24.
Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A practical guide to rodent islet isolation and assessment. Biol Proceed Online. 2009;11:3–31.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, et al. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology. 2001;57:2083–8.
Aslanoglou D, George EW, Freyberg Z. Homogeneous time-resolved forster resonance energy transfer-based assay for detection of insulin secretion. J Vis Exp. 2018;135:e57531.
Farino ZJ, Morgenstern TJ, Vallaghe J, Gregor N, Donthamsetti P, Harris PE, et al. Development of a rapid insulin assay by homogenous time-resolved fluorescence. PLoS ONE. 2016;11:e0148684.
Li X, Wu X, Camacho R, Schwartz GJ, LeRoith D. Intracerebroventricular leptin infusion improves glucose homeostasis in lean type 2 diabetic MKR mice via hepatic vagal and non-vagal mechanisms. PLoS ONE. 2011;6:e17058.
Zaafar DK, Zaitone SA, Moustafa YM. Role of metformin in suppressing 1,2-dimethylhydrazine-induced colon cancer in diabetic and non-diabetic mice: effect on tumor angiogenesis and cell proliferation. PLoS ONE. 2014;9:e100562.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Olofsson CS, Gopel SO, Barg S, Galvanovskis J, Ma X, Salehi A, et al. Fast insulin secretion reflects exocytosis of docked granules in mouse pancreatic B-cells. Pflug Arch. 2002;444:43–51.
Barg S, Lindqvist A, Obermuller S. Granule docking and cargo release in pancreatic beta-cells. Biochem Soc Trans. 2008;36:294–9.
Feldman JM, Chapman B. Characterization of pancreatic islet monoamine oxidase. Metabolism. 1975;24:581–8.
Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, et al. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002;61:729–37.
Khunweeraphong N, Nagamori S, Wiriyasermkul P, Nishinaka Y, Wongthai P, Ohgaki R, et al. Establishment of stable cell lines with high expression of heterodimers of human 4F2hc and human amino acid transporter LAT1 or LAT2 and delineation of their differential interaction with alpha-alkyl moieties. J Pharmacol Sci. 2012;119:368–80.
Camargo SM, Vuille-dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, et al. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. J Pharmacol Exp Ther. 2014;351:114–23.
Sulzer D, Cragg SJ, Rice ME. Striatal dopamine neurotransmission: regulation of release and uptake. Basal ganglia. 2016;6:123–48.
Pivonello R, Ferone D, Lombardi G, Colao A, Lamberts SW, Hofland LJ. Novel insights in dopamine receptor physiology. Eur J Endocrinol. 2007;156:S13–21.
Kongpracha P, Nagamori S, Wiriyasermkul P, Tanaka Y, Kaneda K, Okuda S, et al. Structure-activity relationship of a novel series of inhibitors for cancer type transporter L-type amino acid transporter 1 (LAT1). J Pharmacol Sci. 2017;133:96–102.
Koller WC, Rueda MG. Mechanism of action of dopaminergic agents in Parkinson’s disease. Neurology. 1998;50:S11–14.
Kostrzewa RM. Dopamine receptor supersensitivity. Neurosci Biobehav Rev. 1995;19:1–17.
Lopez Vicchi F, Luque GM, Brie B, Nogueira JP, Garcia Tornadu I, Becu-Villalobos D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharmacol Res. 2016;109:74–80.
Guo N, Guo W, Kralikova M, Jiang M, Schieren I, Narendran R, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology. 2010;35:806–17.
Gangarossa G, Espallergues J, Mailly P, De Bundel D, de Kerchove d’Exaerde A, Herve D, et al. Spatial distribution of D1R- and D2R-expressing medium-sized spiny neurons differs along the rostro-caudal axis of the mouse dorsal striatum. Front Neural Circuits. 2013;7:124.
Armando I, Konkalmatt P, Felder RA, Jose PA. The renal dopaminergic system: novel diagnostic and therapeutic approaches in hypertension and kidney disease. Transl Resd. 2015;165:505–11.
Tayebati SK, Lokhandwala MF, Amenta F. Dopamine and vascular dynamics control: present status and future perspectives. Curr Neurovasc Res. 2011;8:246–57.
Zhang MZ, Harris RC. Antihypertensive mechanisms of intra-renal dopamine. Curr Opin Nephrol Hypertens. 2015;24:117–22.
Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Roson MI, Rodriguez Fermepin M, et al. Renal dopaminergic system: Pathophysiological implications and clinical perspectives. World J Nephrol. 2015;4:196–212.
Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol. 2008;23:2131–46.
Zeng C, Armando I, Luo Y, Eisner GM, Felder RA, Jose PA. Dysregulation of dopamine-dependent mechanisms as a determinant of hypertension: studies in dopamine receptor knockout mice. Am J Physiol Heart Circ Physiol. 2008;294:H551–569.
Morla L, Edwards A, Crambert G. New insights into sodium transport regulation in the distal nephron: Role of G-protein coupled receptors. World J Biol Chem. 2016;7:44–63.
Mitok KA, Freiberger EC, Schueler KL, Rabaglia ME, Stapleton DS, Kwiecien NW et al. Islet proteomics reveals genetic variation in dopamine production resulting in altered insulin secretion. J Biol Chem. 2008;293:5860–5877.
Borelli MI, Rubio M, Garcia ME, Flores LE, Gagliardino JJ. Tyrosine hydroxylase activity in the endocrine pancreas: changes induced by short-term dietary manipulation. BMC Endocr Disord. 2003;3:2.
Vazquez P, Robles AM, de Pablo F, Hernandez-Sanchez C. Non-neural tyrosine hydroxylase, via modulation of endocrine pancreatic precursors, is required for normal development of beta cells in the mouse pancreas. Diabetologia. 2014;57:2339–47.
Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004;56:331–49.
Cheng Q, Beltran VD, Chan SM, Brown JR, Bevington A, Herbert TP. System-L amino acid transporters play a key role in pancreatic beta-cell signalling and function. J Mol Endocrinol. 2016;56:175–87.
Carranza A, Musolino PL, Villar M, Nowicki S. Signaling cascade of insulin-induced stimulation of L-dopa uptake in renal proximal tubule cells. Am J Physiol Cell Physiol. 2008;295:C1602–1609.
Eldrup E, Moller SE, Andreasen J, Christensen NJ. Effects of ordinary meals on plasma concentrations of 3,4-dihydroxyphenylalanine, dopamine sulphate and 3,4-dihydroxyphenylacetic acid. Clin Sci. 1997;92:423–30.
Eldrup E, Richter EA. DOPA, dopamine, and DOPAC concentrations in the rat gastrointestinal tract decrease during fasting. Am J Physiol Endocrinol Metab. 2000;279:E815–22.
Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003;305:800–11.
Goldstein DS, Swoboda KJ, Miles JM, Coppack SW, Aneman A, Holmes C, et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–31.
Eldrup E, Richter EA, Christensen NJDOPA. norepinephrine, and dopamine in rat tissues: no effect of sympathectomy on muscle DOPA. Am J Physiol. 1989;256:E284–287.
Beaulieu JM, Espinoza S, Gainetdinov RR. Dopamine receptors - IUPHAR Review 13. Br J Pharmacol. 2015;172:1–23.
Leggio GM, Bucolo C, Platania CB, Salomone S, Drago F. Current drug treatments targeting dopamine D3 receptor. Pharmacol Ther. 2016;165:164–77.
Newman AH, Beuming T, Banala AK, Donthamsetti P, Pongetti K, LaBounty A, et al. Molecular determinants of selectivity and efficacy at the dopamine D3 receptor. J Med Chem. 2012;55:6689–99.
Ford CP. The role of D2-autoreceptors in regulating dopamine neuron activity and transmission. Neuroscience. 2014;282:13–22.
Mims RB, Scott CL, Modebe OM, Bethune JE. Prevention of L-dopa-induced growth hormone stimulation by hyperglycemia. J Clin Endocrinol Metab. 1973;37:660–3.
Sandyk R. The relationship between diabetes mellitus and Parkinson’s disease. Int J Neurosci. 1993;69:125–30.
Furman BL, Wilson GA. The effects of levodopa on plasma glucose in two strains of rat. Eur J Pharmacol. 1979;55:241–6.
Hahn M, Chintoh A, Giacca A, Xu L, Lam L, Mann S, et al. Atypical antipsychotics and effects of muscarinic, serotonergic, dopaminergic and histaminergic receptor binding on insulin secretion in vivo: an animal model. Schizophr Res. 2011;131:90–95.
Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K. Estimating dopamine D(2) receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33:675–81.
Kopf D, Gilles M, Paslakis G, Medlin F, Lederbogen F, Lehnert H, et al. Insulin secretion and sensitivity after single-dose amisulpride, olanzapine or placebo in young male subjects: double blind, cross-over glucose clamp study. Pharmacopsychiatry. 2012;45:223–8.
Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62:3232–40.
Kowalchuk C, Castellani L, Chintoh A, Remington G, Giacca A, Hahn M. Antipsychotics and glucose metabolism: how brain and body collide. Am J Physiol Endocrinol Metab. 2019;316:E1–15.
Hahn MK, Wolever TM, Arenovich T, Teo C, Giacca A, Powell V, et al. Acute effects of single-dose olanzapine on metabolic, endocrine, and inflammatory markers in healthy controls. J Clin Psychopharmacol. 2013;33:740–6.
Acknowledgements
We gratefully thank and acknowledge Drs. Domenico Accili, Rudolph Leibel, Amy Newman, Marcelo Rubinstein, Lori Sussel, Ruth Singer, Jacob Ballon, Michael McCarthy, Mark Sonders, Nicolas Pierre, Marília Gueiros, Thue Schwartz, Birgitte Holst, Andreas Madsen, Jeffrey Brodsky, and Ms. Emily George for helpful discussions, reagents, and assistance throughout this work. This research was supported by a Department of Defense PRMRP Investigator Initiated Award PR141292 (Z.F.), NIDA K08 DA031241 award (Z.F.), Louis V. Gerstner, Jr., Scholars Program (Z.F.), Leon Levy Foundation (Z.F.), the John F. and Nancy A. Emmerling Fund of The Pittsburgh Foundation (Z.F.), NIDA F32 DA044696 (P.C.D.), NINDS R01 NS075222 (E.V.M.), NIMH R01 MH093672 (C.K.), NINDS Intramural Program (D.R.S., R.B.F.), NIDDK R01 DK116583 (P.E.H.), NARSAD Distinguished Investigator Award (J.A.J.), NIMH R01 MH54137 (J.A.J.), and NIMH T32 MH018870 (J.A.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farino, Z.J., Morgenstern, T.J., Maffei, A. et al. New roles for dopamine D2 and D3 receptors in pancreatic beta cell insulin secretion. Mol Psychiatry 25, 2070–2085 (2020). https://doi.org/10.1038/s41380-018-0344-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0344-6
This article is cited by
-
Antidepressants and type 2 diabetes: highways to knowns and unknowns
Diabetology & Metabolic Syndrome (2023)
-
Short-chain fatty acids: possible regulators of insulin secretion
Molecular and Cellular Biochemistry (2023)
-
Gi/o protein-coupled receptor inhibition of beta-cell electrical excitability and insulin secretion depends on Na+/K+ ATPase activation
Nature Communications (2022)
-
Antipsychotic-induced weight gain and metabolic effects show diurnal dependence and are reversible with time restricted feeding
Schizophrenia (2022)
-
Insulin and glucose metabolism with olanzapine and a combination of olanzapine and samidorphan: exploratory phase 1 results in healthy volunteers
Neuropsychopharmacology (2022)