Abstract
The Alzheimer’s Disease Sequencing Project (ADSP) undertook whole exome sequencing in 5,740 late-onset Alzheimer disease (AD) cases and 5,096 cognitively normal controls primarily of European ancestry (EA), among whom 218 cases and 177 controls were Caribbean Hispanic (CH). An age-, sex- and APOE based risk score and family history were used to select cases most likely to harbor novel AD risk variants and controls least likely to develop AD by age 85 years. We tested ~1.5 million single nucleotide variants (SNVs) and 50,000 insertion-deletion polymorphisms (indels) for association to AD, using multiple models considering individual variants as well as gene-based tests aggregating rare, predicted functional, and loss of function variants. Sixteen single variants and 19 genes that met criteria for significant or suggestive associations after multiple-testing correction were evaluated for replication in four independent samples; three with whole exome sequencing (2,778 cases, 7,262 controls) and one with genome-wide genotyping imputed to the Haplotype Reference Consortium panel (9,343 cases, 11,527 controls). The top findings in the discovery sample were also followed-up in the ADSP whole-genome sequenced family-based dataset (197 members of 42 EA families and 501 members of 157 CH families). We identified novel and predicted functional genetic variants in genes previously associated with AD. We also detected associations in three novel genes: IGHG3 (p = 9.8 × 10−7), an immunoglobulin gene whose antibodies interact with β-amyloid, a long non-coding RNA AC099552.4 (p = 1.2 × 10−7), and a zinc-finger protein ZNF655 (gene-based p = 5.0 × 10−6). The latter two suggest an important role for transcriptional regulation in AD pathogenesis.
Similar content being viewed by others
Introduction
Genomic studies have revealed that late-onset Alzheimer disease (LOAD) is highly polygenic, with as many as 30 susceptibility loci identified through large-scale meta-analysis of genome-wide association studies (GWAS), targeted exome genotyping array, and several early whole exome sequencing (WES) studies [1,2,3,4,5,6,7,8,9,10,11,12]. Although AD susceptibility is highly heritable (h2 = 0.58–0.79) [13], much of its genetic architecture is still unknown and few rare variants have been detected thus far [3, 6, 7, 14,15,16,17,18,19]. Discovery of rare variants in genomic studies, even those with large sample sizes and examining highly heritable diseases, remains challenging due to statistical power limitations in detecting all but the most strongly associated variants (odds ratio (OR) > 1.5) [20,21,22,23]. The protein coding regions of the genome, or exome, are the best characterized and most conserved portions of the genome and the source of most variants identified to date that are responsible for Mendelian diseases [24]; thus, the exome is a more attractive and less expensive target for identifying rare variants of large effect on disease than the non-protein coding portion of the genome.
The Alzheimer’s Disease Sequencing Project (ADSP) was developed jointly by the National Institute on Aging (NIA) and National Human Genome Research Institute (NHGRI) in response to the National Alzheimer’s Project Act milestones (https://aspe.hhs.gov/national-alzheimers-project-act) to fight Alzheimer’s disease (AD) as an effort to analyze the genomes of well-characterized individuals with and without AD. To detect rare variants and genes associated with LOAD, we performed single-variant and gene-based analyses, including annotated loss-of-function analyses, on the ADSP Discovery Phase Case-Control WES dataset, and attempted to replicate associations in three independent WES datasets, a GWAS dataset containing single nucleotide variants (SNVs) that were imputed using the Haplotype Reference Consortium (HRC) [25] reference panel, and the ADSP family-based whole genome sequence dataset.
Methods
Sample selection and data preparation
Study participants were either European-American (EA) or Caribbean Hispanic (CH) ancestry and were sampled in two ways. To maximize contrast between cases and controls, and power to discover novel associations, the majority of participants were chosen using a risk score that included dosages of the APOE ε2/ε3/ε4 alleles, sex and either onset age (for cases) or age at last exam for controls (or pathology-based adjusted age at death for neuropathology control) [26]. All cases were at least 60 years old and met NINCDS-ADRDA criteria for possible, probable or definite AD based on clinical assessment, or had presence of AD (moderate or high likelihood) upon neuropathology examination. To maximize our ability to discover novel genetic associations, we chose cases whose AD risk score indicated that their disease was not well explained by age, sex, or dosages of the APOE ε2/ε3/ε4 alleles. Conversely, cognitively healthy controls were selected with the goal of identifying alleles associated with the increased risk of or protection from late-onset AD. At the time of last exam, all potential controls were at least 60 years old and were either judged to be cognitively normal or did not meet pathological criteria [27, 28] for AD following brain autopsy. Controls were selected for this study on the basis of the risk score indicating that they were the least likely to develop AD by age 85 years. Applying the risk score resulted in a sample that contained 2,220 AD cases (40%) and 752 controls (14%) who were ε4 heterozygotes and 161 AD cases (3%) and 17 controls (<1%) who were ε4 homozygotes.
In addition, we sampled a set of “enriched” cases from families having at least three affected members for whom the diagnosis of AD was verified by direct examination or review of cognitive testing data and medical records. Cases from early-onset AD families or families with a known PSEN1, PSEN2, or APP mutation were excluded. Within each family, we selected only one AD case, typically the member with the lowest a priori AD risk (based on the risk score defined above), provided this person had sufficient genomic DNA. In addition, because 172 of the “enriched” cases described above were of CH ancestry, we also selected a set of 171 age- and sex-matched cognitively normal CH participants to serve as controls. Participant characteristics are shown in Table 1A.
Procedures
Genotype calling and data processing
WES data were generated at the Broad Institute, the Baylor College of Medicine’s Human Genome Sequencing Center, and Washington University’s McDonnell Genome Institute. An effort was made to assign all samples from a study of origin to the same center and there was a relatively balanced number of cases and controls at each center. Genotypes for bi-allelic SNVs and insertion-deletion polymorphisms (indels) were called using ATLAS2 using version Ch37/hg19 of the reference genome. A coordinated effort was implemented for centralized variant calling and quality control (QC) efforts in order to create one batch of data for analysis. Although there were differences in allele frequencies across sequencing centers for some variants, it was difficult to determine whether these represented technical artifacts of different capture kits, variability in genetic background among cohorts assembled for this study, or chance differences that will often occur for infrequent or rare variants. QC steps and methods for evaluating cryptic relatedness, population substructure, differential missingness, and variant annotation are described in the Supplementary Materials.
Single-variant and gene-based association analyses
Statistical models & rationale for covariate adjustments
All models included adjustment for sequencing center and population substructure. Before conducting the primary analyses, we evaluated up to 20 PCs for association with AD status. Only ancestry-specific PCs that significantly associated with AD status (P<0.005) in at least one of the three adjustment models were included as covariates (EA subgroup: PC1, PC5, PC8, PC9, PC10, PC11, PC18; Hispanic analyses included PC1 and PC2). Because most participants for the discovery study were sampled to maximize differences in cases and controls based on age, sex, and APOE genotypes, we included only PCs and sequencing center in our base adjustment model (Model 0). We evaluated two other models that included several covariates in addition to those in the base model: Model 1 adjusted for sex and age at diagnosis or last follow-up; and Model 2 adjusted for APOE ε4 & ε2 dosages in addition to those included in Model 1. All analyses were performed separately by ancestry (EA and CH) using seqMeta (version 1.6) [29]; the primary analysis is an inverse variance-weighted meta-analysis of these two groups. Single variant tests were limited to variants with at least 10 copies of the minor allele across the total QCed sample (MAF~0.0005).
Gene-based association testing
Gene-based tests examine the aggregate effect of risk and protective variants within a region defined by gene annotations. We performed gene-based tests using SKAT-O, which optimally combines SKAT and burden tests [30]. For these analyses, the SKAT portion of the test included variants with a MAF≤0.05; the burden component aggregated variants with MAF≤0.01. The SKAT test used ‘Wu weights’, defined by a beta density function with pre-specified parameters a1 = 1 and a2 = 25, evaluated at the sample minor allele frequency. The SKAT-O statistic, a linear combination between a SKAT statistic (Qskat) and a burden statistic (Qburden) equal to (1-ρ) Qskat + ρQburden, was optimized across 11 values of ρ (0.1 increments), and calculation of the significance took into consideration the multiple values of ρ evaluated. In order to improve power by removing variants predicted to have a low functional impact on the translated protein, we filtered variants in each gene on the basis of annotated function as described in the Supplementary Materials. We performed SKAT-O testing for genes with at least two qualifying variants contributing to the test. The minimum number of aggregated alleles (i.e., cumulative minor allele count or cMAC) for a gene-based test was set at 10.
Statistical significance thresholds for discovery stage analyses
Within each analysis framework including individual variants and gene-based aggregation of variants evaluated under particular functional annotation criteria, suggestive associations (p < 1/ # tests) were selected for follow-up testing in independent samples and a Bonferroni-corrected threshold was used to define experiment wide statistical significance (p < 0.05 / # tests). We did not correct for the three models and meta-analyses of the combined results of the EA + CH populations because the results were highly correlated across the covariate adjustment strategies (Supplementary Figure S2).
Replication sample and analyses
Primary replication analyses for the SNVs / genes that we identified to be genome-wide significant or suggestive in any model were conducted in three independent WES datasets including CHARGE (612 cases and 1,836 controls), ADES-FR (1,142 cases and 1,104 controls) [31], and FinnAD (1,024 cases and 4,322 controls), as well as in the Alzheimer’s Disease Genetics Consortium (ADGC) HRC-imputed GWAS dataset (Table 1B, Supplementary Materials, Supplementary Table S1). The ADGC dataset included GWAS data on 9,343 cases and 11,527 cognitively normal elders from 32 datasets for whom genotypes were imputed using the Haplotype Reference Consortium (HRC) r1.1 reference panel (Supplementary Table S2) [25, 32]. CHARGE and ADGC participants selected for ADSP discovery analyses were not included in the replication study.
Because we included all available cases and controls in the replication datasets instead of applying the participant selection criteria used for the discovery sample to maximize difference in cases and controls, model 0 is not appropriate in replication studies. Hence, single variant tests and gene-based SKAT-O tests were performed using seqMeta for models 1 and 2 only. Meta-analysis of summarized results from the four samples was performed using seqMeta. We also performed a meta-analysis of results across the ADSP discovery and four replication cohorts for findings that were at least “suggestive” (p < 1 / genes or variants) in the discovery phase. In addition to models 1 and 2, we conducted a meta-analysis of results obtained using model 0 in the ADSP discovery data and model 1 in the replication cohorts to verify our findings in ADSP model 0. Because the ADSP discovery dataset includes CH participants and all replication cohorts consist of EA participants only, we performed the meta-analysis with and without CH participants in ADSP. We considered any variants or genes with two-stage meta-analysis p-values < 0.05 / # tests to be significant per the recommendation by Skol et al. that joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies [33]. We acknowledge, however, that additional replication in independent samples is required.
The top findings in the discovery sample were also followed-up in the ADSP whole-genome sequenced (WGS) family-based dataset [34, 35]. This dataset includes 197 individuals sequenced in 42 EA families and 351 individuals in 67 CH families. Additional follow-up was also performed using WGS information from 150 members of another 48 CH families. Individual variants were assessed by examining their co-segregation with AD status within families. Gene-based association tests were performed using the FSKAT software [36].
Analysis of variants at previously established AD loci
To identify a set of variants related to AD risk in loci previously associated with AD, we compiled a list of genes containing variants with significant or suggestive associations (p < 1 × 10−3) in either the published IGAP or UKBB AD GWAS meta-analyses [9, 37]. Because many signal variants from GWAS are in intergenic regions, we used a combination of BEDOPS [38] and BEDTools [39] operations to enlarge the genomic coordinates of these associated variants by 50 Kb on each side, merging adjacent regions that were overlapping and/or book-ended. Of the resulting genomic regions, segments greater than 100 kb were retained, shortened by 50 kb on each side, merged if separated by 200 kb or less, and utilized to find overlapping protein-coding genes, with gene boundaries as defined in version 19 of the GENCODE gene set [40] and a 50 kb buffer on each side. These parameters and sequence of operations were chosen because they resulted in an algorithm that satisfactorily captured the genomic interval of the association landscape at each locus, as confirmed by visual inspection of LocusZoom regional plots [41]. We queried variant and gene level association statistics for the resulting list of 299 putatively associated AD genes.
Results
Description of study samples after QC and filtering
After exclusions, 10,836 participants were available for analysis (5,740 cases; 5,096 controls). This included 218 CH cases and 177 CH controls. The study included more women than men, and, due largely to the selection criteria, cases were younger on average than controls and were more likely to carry one copy of the APOE ε4 allele. In total, the data included 1,524,414 bi-allelic SNVs or short indels. Most variants were rare, with 1,493,926 (98%) of variants having minor allele frequency of less than 5% and 160,898 (11%) having a minor allele count (MAC) of at least 10 copies.
Single-variant SNV and short indels association analysis
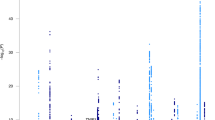
We performed single variant analyses for the 160,898 variants with a combined minor allele count of at least 10 copies across all participants (Supplementary Figure S3). Genomic inflation was moderate (λ < 1.1 in all models) (Supplementary Figures S4–S7). Single variant association testing identified three variants at an exome-wide significance level (p < 3.1 × 10−7) and 14 variants at the suggestive threshold (p < 6.1 × 10−6) outside of the APOE region (Table 2, Fig. 1, Supplementary Table S3). The significant associations included the rare missense R47H variant in TREM2 (rs75932628, p = 4.8 × 10−12), a common variant in PILRA (rs2405442, p = 1.7 × 10−7), and a novel rare variant in the long non-coding RNA AC099552.4 (7:154,988,675, p = 1.2 × 10−7). These results were attenuated when including age, sex, and APOE ε2/ε3/ε4 allele as covariates.
Manhattan plot showing genome-wide association results for individual common variants. The plot shows the p-values from the Discovery meta-analysis against their genomic position for association with AD. Only variants with a combined minor allele count of ≥ 10 were included; the minimum p-value from the three adjustment models for either the meta-analysis, European Ancestry (EA), or Caribbean Hispanic (CH) is plotted for each variant. Genes containing the variant are indicated above points that surpassed our significance threshold for follow-up. The dotted line indicates the threshold for follow-up, p < 6.1 × 10−6, corresponding to (1 / #variants) tested. The dashed line indicates the threshold for exome-wide significance, p < 3.1 × 10−7, corresponding to (0.05 / #variants tested)
Gene-based association analysis combining SNVs and indels
We aggregated 918,053 variants with a combined MAF < 0.05 and annotated as high or moderate impact into gene-based tests using SKAT-O. This corresponds to 17,613 genes with more than one variant and a cumulative minor allele count (cMAC) of at least 10 copies. Applying more stringent filtering, we limited to variants annotated as high impact; aggregating 42,502 rare or uncommon (MAF < 0.05) variants into 4,634 genes (again, limiting to genes with >1 variant and a cMAC ≥ 10). For the purposes of identifying novel associations, we considered all genes or variants within 250kb of APOE as part of the APOE locus. Three known genes (ABCA7, TREM2 and CBLC in the APOE region) and two novel genes (OPRL1 and GAS2L2) achieved exome-wide statistical significance for their respective tests in the discovery analyses (Table 3, Fig. 2). Four additional genes (ZNF655, RHBDD1, SIRPB1, and RPS16) reached suggestive significance across the nine models (Fig. 2, Supplementary Table S4). Analyses filtered to include only variants with CADD scores ≥ 15 or ≥ 20 produced most of the same top-ranked results as the VEP gene-based results (Supplementary Table S5), noting that the overall VEP High/Moderate and CADD≥15 results, as well as the VEP High and CADD≥20 results, are only moderately correlated (Spearman rank correlation r = 0.51) (Supplementary Figure S8). Three novel genes (CACNB3, HHIP-AS1, and RP11-68L1.1) were exome-wide statistically significant in the CH group in analyses restricted to variants with CADD scores ≥ 20 (Supplementary Table S5), however these are likely false positives because in each instance the result is accounted for by a single variant that was observed in one person only.
Manhattan plots showing exome-wide association results for gene-based tests of rare functional variants. The plots show the gene-based p-values from the Discovery meta-analysis against their genomic position for association with AD. Each point represents a p-value from SKAT-O test aggregating rare variants (MAF < 5%), by gene, on the basis of predicted functional impact. Only genes with a cumulative minor allele count of ≥ 10 were included; the minimum p-value from the three adjustment models for either the meta-analysis, European Ancestry (EA), or Caribbean Hispanic (CH) is plotted for each variant. Genes are indicated above points that surpassed our significance threshold for follow-up in tests aggregating only (a) moderate or high impact variants, (b) high impact variants; (c) loss-of-function variants. In each plot, the dotted line indicates the threshold for follow-up: (a) p<5.5 × 10−5, (b) p<6.3 × 10−5, (c) p<2.8 × 10−4, each corresponding to 1 / # genes tested. The dashed line indicates the threshold for exome-wide significance: (a) p<2.7 × 10−6, (b) p<3.1 × 10−6, (c) p < 1.4 × 10−5, each corresponding to 0.05 / # genes tested
Loss-of-function (LOF) association analysis
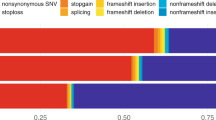
Among 78,529 unfiltered high impact variants, 72,694 were annotated as LoF and 68,121 were further deemed as high-confidence, most of which were frameshift and stop-gained (Fig. 3). As expected, over 90% of these high-confidence LoF variants were singletons (53,120, 78%), doubletons (6,579, 10%), or tripletons (2,222, 3%), and most of these were observed in European Americans only. Association analysis of 2,378 high-confidence LoF variants with MAC ≥ 10 with adjustment for sequencing center and PCs revealed one Bonferroni corrected significant p < 2.1 × 10−5) variant, a previously reported frameshift deletion in ABCA7 (Table 3) [42]. Gene-based analysis of 32,863 high-confidence LoF variants with MAF ≤ 5% mapping to 3,558 genes with at least two variants and cMAC ≥ 10 (Supplementary Table S4) also showed that ABCA7 with adjustment for sequencing center and PCs (Model 0) and GAS2L2 with adjustment for sequencing center, PCs, sex, age, and APOE genotype (Model 2) reached experiment-wide significance threshold p < 1.4 × 10−5).
Replication analysis
Of the 16 single variants outside the APOE region tested in the replication samples (Table 2, Supplementary Table S3), the TREM2 R47H mutation and four variants in three other previously known genes (one missense and one synonymous variant in MS4A6A, a synonymous variant in PILRA, and a missense variant in CR1) were significantly associated with AD in the combined discovery and replication analysis (Table 2). Associations with two variants in a novel gene STAG3 (rs1043915, p = 5.5 × 10−6) were also replicated and significantly associated with AD. We were unable to assess replication with the novel AC099552.4 variant because it was not observed or imputed in the replication datasets. One of the IGHG3 variants (rs12890621) showed borderline evidence for association in models 1 and 2 (p = 0.085 and 0.075, respectively), and evidence for association was strengthened to near exome-wide significance (p = 9.8 × 10−7) in the combined discovery and replication sample.
In total, 19 genes across the nine models were significantly or suggestively associated with AD and were tested in the replication stage (Supplementary Table S4). Gene-based tests including high or moderate impact variants showed evidence for replication and reached genome-wide significance in the combined discovery and replication analysis for three genes:TREM2 and two genes in the APOE region (CBLC and BCAM) (Table 3, Supplementary Table S6). The association with GAS2L2, the potential novel gene identified in a model 2 SKAT-O test with high impact SNVs in the discovery sample, was slightly above the nominal significance level (p = 0.051 and p = 0.067, respectively, in models 1 and 2) in meta-analysis across four replication cohorts. However, this association was only nominally significant in the meta-analysis combining the discovery and replication cohorts (p = 0.029 for model 2).
In gene-based tests including only high impact SNVs, the known AD risk gene ABCA7 and the potential novel gene ZNF655 reached the nominal p-value of 0.05 in meta-analysis with replication cohorts as well as in a meta-analysis of discovery and replication samples (Table 3, Supplementary Table S6). These two genes were also nominally significant in SKAT-O tests, limited to high impact variants (ZNF655: p = 7.9 × 10−6; ABCA7: p = 6.2 × 10−5) and LOF variants (ZNF655: p = 5.0 × 10−6).
Because PILRA, a previously established AD gene [43] is proximate to STAG3 (159 kb) and ZNF655 (797 kb), we performed conditional analysis in the discovery sample to determine whether these novel association signals are independent. These analyses demonstrated that the association with multiple rare variants ZNF655 in the gene-based test is distinct from those with common variants in PILRA (p = 1.08 × 10−4) and STAG3 (p = 7.75 × 10−5). In a model containing both PILRA and STAG3 variants the association with PILRA remains significant (p = 0.011) but the association with STAG3 does not (p = 0.21) (Supplementary Table S7).
Follow-up in ADSP family-based data
We also followed-up the significant and suggestive single-variant and gene-based results from the discovery stage in the ADSP whole-genome sequenced (WGS) family-based dataset. A rare missense variant (rs61756195, MAF = 0.001) in STAG3 segregated with disease in three CH families and trended toward association in the case-control study (p = 0.052) (Supplementary Table S8). Gene-based testing identified a nominal association for GAS2L2 (p = 0.049) in EA families (Supplementary Table S9).
Rare variants in established genes from GWAS
We interrogated our individual variant and gene-based aggregate association tests for 299 previously associated AD genes. Among the SNVs and indels, a total of 1,172 variants with MAF < 0.05 and annotated as either HIGH or MODERATE impact are located within 253 interrogated AD genes (Supplementary Table S10). Five of these variants were at least suggestively significant (p<8.9 × 10−4) in single variant testing. The most significant associations included the TREM2 R47H missense mutation (p = 4.8 × 10−12) and ABCA7 frameshift mutation E709fs (p = 4.3 × 10−6) which was previously associated with AD in Belgian families [40]. Additional notable signals included variants in SORL1 A528T (p = 8.7 × 10−5), which was previously associated with AD in a CH population [15], and ACP2 D353E (p = 7.8 × 10−4). Perturbation of murine Acp2 causes lysosomal storage deficits, kyphoscoliosis, cerebellar abnormalities, and ataxia [44, 45].
For gene-based tests, we aggregated variants on the basis of annotated function, and examined only genes with more than one contributing variant and a cMAC ≥ 10. Of the 299 AD genes, tests were performed on 281 genes aggregating high or moderate impact variants and 86 genes limited to high impact variants. Among these, 13 unique genes surpassed suggestive significance thresholds for high (p < 1.16 × 10−2)orhigh-moderate (p = 3.56 × 10−3) impact variants. The strongest associations were observed for moderate impact variants in TREM2 (p = 4.81 × 10−12) and SORL1 (p = 8.68 × 10−5), and a high impact variant in ABCA7 (p = 4.33 × 10−6). Other noteworthy signals included moderate impact variants in NUP88 (p = 4.63 × 10−4) and ACP2 (p = 7.80 × 10−4).
Discussion
Our WES study, the largest for AD conducted to date, identified novel associations with variants in three genes not previously implicated in AD including one common nearly exome-wide significant variant each in IGHG3 (p = 9.8 × 10−7) and STAG3 (p = 8.8 × 10−7), and one rare exome-wide significant variant in AC099552.4 (p = 1.2 × 10−7). We also observed a gene-wide significant association with ZNF655 in a gene-based test including nine high-impact rare variants (p = 5.0 × 10−6). These results remained significant after multiple test correction and were confirmed in or strengthened by a replication sample comprised of four independent datasets, with the exception of the variant in AC099552.4 which was invariant in the replication samples. We also confirmed associations with common and rare variants in several previously established AD genes including ABCA7, APOE, HLA-DPA1, MS4A6A, PILRA, SORL1 and TREM2.
ZNF655 is expressed in brain and encodes the Vav-interacting Krüppel-like factor 1 [46]. Krüppel-like factors (KLFs) are zinc finger-containing transcription factors that regulate diverse biological processes, including proliferation, differentiation, growth, development, survival, and responses to external stress [47]. Several KLFs have been shown to participate in neuronal morphogenesis and to control the regenerative capacity of neurons in the central nervous system. AC099552.4 is a long non-coding RNA, an abundant class of RNA sequences which regulate gene transcription and expression [48] and impact neuronal development, neuroplasticity, and cognition [49]. Non-coding RNA-dependent regulation affecting AD-related processes has been demonstrated for SORL1 [50] and in a triple transgenic model of AD [51].
IGHG3 encodes immunoglobulin heavy constant gamma 3 and is a member of the IgG family for which antibodies have been shown to cross-react with fibril and oligomer amyloid-β aggregates [52] leading to speculation that Immunoglobulin GM (γ marker) genes contain functional risk and protective factors for AD [53]. The anti-amyloidogenic activity of IgG appears to be an inherent property of free human IgG heavy chains [54]. Recent analysis of structural variants in whole genome sequence data for 578 members of 101 families with multiple AD subjects included in the ADSP [26] yielded additional evidence supporting IGHG3 as an AD risk locus. A total of nine distinct deletions in the IGH region were identified as disproportionately represented in AD cases compared to controls. One of these is a 188 bp deletion that was observed in 35 AD cases and 8 controls and is located 592 bp from the AD-associated SNV (rs12890621) in this study. This deletion eliminates a large portion of IGHG3 intron 2 and exon 3 (reference transcript ENST00000390551), and is predicted to have high impact on the encoded product. It is unlikely that the deletion and rs12890621 tag the same effector of AD risk because the deletion is rare, whereas rs12890621 is more common (MAF = 0.0475 in EA subjects according to the ExAC database). Of note, a nearby pseudogene in the IgG family, IGHV1-67, located approximately 350 kb from IGHG3, has been previously reported in a gene-wide association study conducted by the International Genetics of Alzheimer’s Project (IGAP) [1].
The association with the common synonymous variant in STAG3 (rs1043915, MAF = 0.26) is not independent of the finding with a common SNV in PILRA, a previously reported AD-associated gene [43] located in an established AD locus [9]. However, rare variants in STAG3 identified by WGS showed evidence of co-segregation with AD in CH families suggesting the possibility that STAG3 has a distinct mechanistic role in AD. STAG3, stromal antigen 3, encodes a subunit of the cohesin complex which regulates the cohesion of sister chromatids during cell division. Whether the association with AD observed here is mediated at least in part through STAG3 function or simply reflects linkage disequilibrium with other causal variants/genes in the region remains to be established. Rare coding STAG3 variants have been identified in primary ovarian insufficiency [55]. Although STAG3 is expressed in the brain, its role remains unclear. Interestingly, data from GTEx show that the associated variant is an eQTL for multiple genes in various brain tissues, including STAG3, AGFG2, GAL3ST4, GATS, and PVRIG. In a mouse model of diabetes, microvascular damage in the neurovascular unit of the retina was associated with alteration in STAG3 expression [56].
A variant in NSF showed nominally significant evidence of association in the replication sample (p = 0.014) in the model adjusting for age, sex, and APOE ε4 status, whereas the result in the discovery sample was observed in the model without these covariates. NSF encodes N-ethylmaleimide sensitive factor, vesicle fusing ATPase is involved in membrane trafficking of proteins and neurotransmitter release [57], and has been observed in brain homogenates of cases of familial neuronal intranuclear inclusion disease [58]. NSF SNVs have been associated with cocaine dependence [59] and its expression is reduced in prefrontal cortex in schizophrenia patients [60]. Vesicular trafficking has an important role in AD exemplified by genetic and biological evidence for neuronal sorting proteins including SORL1 [61,62,63].
We were unable to replicate variants at five loci that showed significant association in the discovery sample (p ≤ 5.0 × 10−6). Failure to replicate findings for the OPRL1 and DTNBP1 variants may be due to their lower MAF and, hence, uncertainty in the imputation quality and lack of imputed indels in the ADGC GWAS replication sample. Nonetheless, both of these genes are potentially attractive biological candidates. Opioid related nociceptin receptor 1 modulates a variety of biological functions and neurobehavior, including learning and memory, and inflammatory and immune functions [64, 65]. DTNBP1 encodes the dystrobrevin binding protein 1 which has been genetically linked to multiple psychiatric disorders, as well as cognitive and memory functions in healthy human subjects [66, 67].
Analysis of rare variants in the regions of genes previously identified as related to AD by GWAS revealed genome-wide significant or suggestive evidence of association in established genes including TREM2, SORL1, and ABCA7. In addition, notable associations were observed with other genes in these regions not previously linked to AD including TREML4, SPPL2A, and AP4M1 (Supplementary Table S10). TREML4 is located near TREM2 and encodes a TREM family receptor that, similar to TREM2, is expressed on the surface of myeloid cells and participates in the phagocytic clearance of dead cells [68]. SPPL2A encodes an endosomal-lysosomal protease and presenilin homolog that regulates B-cell homeostasis in vivo [69]. Homozygous mutations in AP4M1, located in the region including PILRA and STAG3, cause spastic tetraplegia, intellectual disability, and white matter loss [70]. Its encoded protein is a component of the AP-4 trafficking complex that regulates APP processing and beta-amyloid secretion in cell models [71]. Further studies are needed to conclude whether the association findings in this latter group of genes are robust and warrant experiments to determine their functional relevance to AD.
Notably, there is little overlap of our results with findings of large GWAS focused on common variants [1, 2, 9]. This is due in part to our focus on only infrequent or rare variants (MAF < 0.05) that are functionally-annotated to be of at least moderate impact and may not have been well covered by GWAS arrays or imputation. With the notable exception of APOE, common variants associated with AD have very modest effect on risk (OR < 1.3) [9], and all but a few of these associations [4, 5, 8, 10, 12] required a sample between two and nearly seven times larger than the sample in this study to have sufficient power to detect them [1, 2, 9].
Our study has several notable strengths and limitations. The ascertainment scheme for this sample is optimal for detection of association with both risk and protective variants for AD [26]. Specifically, the AD cases were selected to have relatively early onset (with a minimum age of 65) and a lower frequency of the APOE ε4 allele with the expectation that they were likely to be more enriched for rare high-penetrant AD risk-variants compared to most late-onset AD cases. Controls were selected to be as old as possible with preference given to those having at least one APOE ε4 allele to enrich this group for protective variants. However, this scheme introduced confounding between age and AD status which reduced power for detecting associations. To overcome this limitation, we included a model without age adjustment which yielded the largest number of new association findings including several that were replicated in independent datasets which were analyzed with age adjustment. Thus, it was important to include models which did or did not include a covariate for age in order to account for confounding with AD status as well as age-dependent effects of the genetic factor. Despite simulations showing that this sample had sufficient power to detect associations with variants whose frequencies were as low as 0.005 and an effect size greater than 1.8 [26], the number of novel rare variant findings were few. We also acknowledge that p-value thresholds did not account for the number of models tested, however the models are highly correlated (Supplementary Table S2).
The inclusion of CH participants who were a pivotal portion of a multi-ethnic sample leading to the discovery of common variant associations in other AD loci, most notably SORL1 [62], but for rare variant discovery these samples may have reduced power by increasing genetic heterogeneity of the total sample. This conclusion is consistent with observations of few novel findings in this WES study showing discernable contributions by the CH dataset and by discovery of novel rare variant associations in a whole genome sequence study that were unique to EA and CH families, respectively [34, 35]. Nonetheless, the non-Hispanic portion of our sample was sufficiently large to detect multiple novel associations. Our findings suggest that additional large and ancestrally diverse cohorts with deep sequence data will need to be examined for replication and to provide a larger discovery sample.
Successful replication of only some of the most significant findings in novel genes not in the APOE region (4/8 individual variants in Table 2, 1/18 genes in Table 3) is somewhat concerning but highlights the difficulty of designing well-powered replication studies of sequencing findings. Although it is possible that some of these findings are false positives, we acknowledge that the size of the WES replication samples combined (2,778 AD cases, 7,262 controls) was inadequate. In addition, many rare variants were not well-imputed or, in the case of most indels, not imputed at all in the ADGC GWAS dataset, despite the use of the HRC reference panel which contains haplotypes derived from whole genome sequence data for more than 30,000 individuals who were not ascertained for AD research. Thus, additional large WES samples will need to be studied to obtain definitive evidence about findings that did not replicate.
In summary, our significant association findings with functional rare variants in novel genes provide further support for the roles of neuroinflammation (IGHG3) and transcriptional regulation (AC099552.4 and ZNF655) in AD. In addition, we identified many novel associations with rare functional variants in previously established AD genes. In most cases, these rare variants do not explain association signals that were previously identified by GWAS with common and predominantly non-functional variants. Hence, many of our findings will provide insight into disease mechanisms and targets for biological experiments to gain further understanding about the role of these genes in AD pathogenesis. However, other deep sequencing approaches (e.g., whole genome, target gene resequencing) will be needed to identify variants which account for association signals in non-coding regions and the contribution of structural variants (e.g., larger insertions and deletions, copy number variants, etc.) to AD risk.
Change history
21 October 2019
A Correction to this paper has been published: https://doi.org/10.1038/s41380-019-0529-7
References
Escott-Price V, Bellenguez C, Wang LS, Choi SH, Harold D, Jones L, et al. Gene-wide analysis detects two new susceptibility genes for Alzheimer's disease. PLoS One. 2014;9:e94661.
Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer's disease. Brain. 2015;138:3673–84.
Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. TREM2 variants in Alzheimer's disease. N Engl J Med. 2013;368:117–27.
Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshere ML, et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer's disease. Nat Genet. 2009;41:1088–93.
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43:429–35.
Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–99.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368:107–16.
Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009;41:1094–9.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–8.
Naj AC, Jun G, Beecham GW, Wang LS, Vardarajan BN, Buros J, et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer's disease. Nat Genet. 2011;43:436–41.
Ruiz A, Heilmann S, Becker T, Hernandez I, Wagner H, Thelen M, et al. Follow-up of loci from the International Genomics of Alzheimer's Disease Project identifies TRIP4 as a novel susceptibility gene. Transl Psychiatry. 2014;4:e358.
Seshadri S, Fitzpatrick AL, Ikram MA, DeStefano AL, Gudnason V, Boada M, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–40.
Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry. 2006;63:168–74.
Vardarajan BN, Ghani M, Kahn A, Sheikh S, Sato C, Barral S, et al. Rare coding mutations identified by sequencing of Alzheimer disease genome-wide association studies loci. Ann Neurol. 2015;78:487–98.
Vardarajan BN, Zhang Y, Lee JH, Cheng R, Bohm C, Ghani M, et al. Coding mutations in SORL1 and Alzheimer disease. Ann Neurol. 2015;77:215–27.
Steinberg S, Stefansson H, Jonsson T, Johannsdottir H, Ingason A, Helgason H, et al. Loss-of-function variants in ABCA7 confer risk of Alzheimer's disease. Nat Genet. 2015;47:445–7.
Logue MW, Schu M, Vardarajan BN, Farrell J, Bennett DA, Buxbaum JD, et al. Two rare AKAP9 variants are associated with Alzheimer's disease in African Americans. Alzheimers Dement. 2014;10:609–18.
Jun G, Asai H, Zeldich E, Drapeau E, Chen C, Chung J, et al. PLXNA4 is associated with Alzheimer disease and modulates tau phosphorylation. Ann Neurol. 2014;76:379–92.
Wetzel-Smith MK, Hunkapiller J, Bhangale TR, Srinivasan K, Maloney JA, Atwal JK, et al. A rare mutation in UNC5C predisposes to late-onset Alzheimer's disease and increases neuronal cell death. Nat Med. 2014;20:1452–7.
Bodmer W, Bonilla C. Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet. 2008;40:695–701.
Schork NJ, Murray SS, Frazer KA, Topol EJ. Common vs. rare allele hypotheses for complex diseases. Curr Opin Genet Dev. 2009;19:212–9.
Surakka I, Horikoshi M, Magi R, Sarin AP, Mahajan A, Lagou V, et al. The impact of low-frequency and rare variants on lipid levels. Nat Genet. 2015;47:589–97.
Pritchard JK. Are rare variants responsible for susceptibility to complex diseases? Am J Hum Genet. 2001;69:124–37.
Rabbani B, Tekin M, Mahdieh N. The promise of whole-exome sequencing in medical genetics. J Hum Genet. 2014;59:5–15.
McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet. 2016;48:1279–83.
Beecham GW, Bis JC, Martin ER, Choi S-H, DeStefano A, van Duijn C, et al. The Alzheimer’s Disease Sequencing Project: study design and sample selection. Neurol Genet. 2017;3:e194.
Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer's disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–44.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Lumley T, Brody J, Dupuis J, Cupples A Meta-analysis of a rare-variant association test: University of Auckland; 2012. http://stattech.wordpress.fos.auckland.ac.nz/files/2012/11/skat-meta-paper.pdf. Technical report.
Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA, et al. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am J Hum Genet. 2012;91:224–37.
Bellenguez C, Charbonnier C, Grenier-Boley B, Quenez O, Le Guennec K, Nicolas G, et al. Contribution to Alzheimer's disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging. 2017;59:220 e221–220 e229.
Loh PR, Danecek P, Palamara PF, Fuchsberger C, A Reshef Y, K Finucane H, et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet. 2016;48:1443–8.
Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38:209–13.
Blue EE, Bis JC, Dorschner MO, Tsuang D, Barral SM, Beecham G, et al. Genetic variation in genes underlying diverse dementias may explain a small proportion of cases in the Alzheimer’s Disease Sequencing Project. Dement Ger Cog Disorders. 2018;45:1–17. 45
Beecham GW, Vardarajan BN, Blue E, Barral S, Haines JL, Bush WS, et al. Whole-genome sequencing in familial late-onset Alzheimer's disease identifies variation in AD candidate genes. Alzheimer Dement. 2017;13:P571–P572.
Yan Q, Tiwari HK, Yi N, Gao G, Zhang K, Lin WY, et al. A sequence kernel association test for dichotomous traits in family samples under a generalized linear mixed model. Hum Hered. 2015;79:60–68.
Liu JZ, Erlich Y, Pickrell JK. Case-control association mapping by proxy using family history of disease. Nat Genet. 2017;49:325–31.
Neph S, Kuehn MS, Reynolds AP, Haugen E, Thurman RE, Johnson AK, et al. BEDOPS: high-performance genomic feature operations. Bioinformatics. 2012;28:1919–20.
Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2.
Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–74.
Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7.
Cuyvers E, De Roeck A, Van den Bossche T, Van Cauwenberghe C, Bettens K, Vermeulen S, et al. Mutations in ABCA7 in a Belgian cohort of Alzheimer's disease patients: a targeted resequencing study. Lancet Neurol. 2015;14:814–22.
Logue MW, Schu M, Vardarajan BN, Farrell J, Lunetta KL, Jun G, et al. A search for genetic risk variants for age-related macular degeneration in Alzheimer disease genes and pathways. Neurobiol Aging. 2014;35:1510.e7–e1510.e18.
Saftig P, Hartmann D, Lullmann-Rauch R, Wolff J, Evers M, Koster A, et al. Mice deficient in lysosomal acid phosphatase develop lysosomal storage in the kidney and central nervous system. J Biol Chem. 1997;272:18628–35.
Mannan AU, Roussa E, Kraus C, Rickmann M, Maenner J, Nayernia K, et al. Mutation in the gene encoding lysosomal acid phosphatase (Acp2) causes cerebellum and skin malformation in mouse. Neurogenetics. 2004;5:229–38.
Houlard M, Romero-Portillo F, Germani A, Depaux A, Regnier-Ricard F, Gisselbrecht S, et al. Characterization of VIK-1: a new Vav-interacting Kruppel-like protein. Oncogene. 2005;24:28–38.
McConnell BB, Yang VW. Mammalian Kruppel-like factors in health and diseases. Physiol Rev. 2010;90:1337–81.
Goodrich JA, Kugel JF. Non-coding-RNA regulators of RNA polymerase II transcription. Nature Rev Molec Cell Biol. 2006;7:612–6.
Butler AA, Webb WM, Lubin FD. Regulatory RNAs and control of epigenetic mechanisms: expectations for cognition and cognitive dysfunction. Epigenomics. 2016;8:135–51.
Ciarlo E, Massone S, Penna I, Nizzari M, Gigoni A, Dieci G, et al. An intronic ncRNA-dependent regulation of SORL1 expression affecting Aβ formation is upregulated in post-mortem Alzheimer's disease brain samples. Dis Model Mech. 2013;6:424–33.
Lee DY, Moon J, Lee ST, Jung KH, Park DK, Yoo JS, et al. Distinct expression of long non-coding RNAs in an Alzheimer's disease model. J Alzheimers Dis. 2015;45:837–49.
O'Nuallain B, Acero L, Williams AD, Koeppen HP, Weber A, Schwarz HP, et al. Human plasma contains cross-reactive Abeta conformer-specific IgG antibodies. Biochemistry. 2008;47:12254–6.
Pandey JP. Immunoglobulin GM genes as functional risk and protective factors for the development of Alzheimer's disease. J Alzheimers Dis. 2009;17:753–6.
Adekar SP, Klyubin I, Macy S, Rowan MJ, Solomon A, Dessain SK, et al. Inherent anti-amyloidogenic activity of human immunoglobulin gamma heavy chains. J Biol Chem. 2010;285:1066–74.
He WB, Banerjee S, Meng LL, Du J, Gong F, Huang H, et al. Whole-exome sequencing identifies a homozygous donor splice-site mutation in STAG3 that causes primary ovarian insufficiency. Clin Genet 2018;93-340–4.
Friedrichs P, Schlotterer A, Sticht C, Kolibabka M, Wohlfart P, Dietrich A, et al. Hyperglycaemic memory affects the neurovascular unit of the retina in a diabetic mouse model. Diabetologia. 2017;60:1354–8.
Pittaluga A, Feligioni M, Longordo F, Luccini E, Raiteri M. Trafficking of presynaptic AMPA receptors mediating neurotransmitter release: neuronal selectivity and relationships with sensitivity to cyclothiazide. Neuropharmacology. 2006;50:286–96.
Pountney DL, Raftery MJ, Chegini F, Blumbergs PC, Gai WP. NSF, Unc-18-1, dynamin-1 and HSP90 are inclusion body components in neuronal intranuclear inclusion disease identified by anti-SUMO-1-immunocapture. Acta Neuropathol. 2008;116:603–14.
Fernandez-Castillo N, Cormand B, Roncero C, Sanchez-Mora C, Grau-Lopez L, Gonzalvo B, et al. Candidate pathway association study in cocaine dependence: the control of neurotransmitter release. World J Biol Psychiatry. 2012;13:126–34.
Imai C, Sugai T, Iritani S, Niizato K, Nakamura R, Makifuchi T, et al. A quantitative study on the expression of synapsin II and N-ethylmaleimide-sensitive fusion protein in schizophrenic patients. Neurosci Lett. 2001;305:185–8.
Reitz C, Cheng R, Rogaeva E, Lee JH, Tokuhiro S, Zou F, et al. Meta-analysis of the association between variants in SORL1 and Alzheimer disease. Arch Neurol. 2011;68:99–106.
Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–77.
Willnow TE, Andersen OM. Sorting receptor SORLA-a trafficking path to avoid Alzheimer disease. J Cell Sci. 2013;126:2751–60.
Gavioli EC, de Medeiros IU, Monteiro MC, Calo G, Romao PR. Nociceptin/orphanin FQ-NOP receptor system in inflammatory and immune-mediated diseases. Vitam Horm. 2015;97:241–66.
Abdel-Mouttalib O. Nociceptin/orphanin-FQ modulation of learning and memory. Vitam Horm. 2015;97:323–45.
Hashimoto R, Noguchi H, Hori H, Nakabayashi T, Suzuki T, Iwata N, et al. A genetic variation in the dysbindin gene (DTNBP1) is associated with memory performance in healthy controls. World J Biol Psychiatry. 2010;11:431–8.
Hashimoto R, Noguchi H, Hori H, Ohi K, Yasuda Y, Takeda M, et al. Association between the dysbindin gene (DTNBP1) and cognitive functions in Japanese subjects. Psychiatry Clin Neurosci. 2009;63:550–6.
Hemmi H, Idoyaga J, Suda K, Suda N, Kennedy K, Noda M, et al. A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J Immunol. 2009;182:1278–86.
Mentrup T, Fluhrer R, Schroder B. Latest emerging functions of SPP/SPPL intramembrane proteases. Eur J Cell Biol. 2017;96:372–82.
Verkerk AJ, Schot R, Dumee B, Schellekens K, Swagemakers S, Bertoli-Avella AM, et al. Mutation in the AP4M1 gene provides a model for neuroaxonal injury in cerebral palsy. Am J Hum Genet. 2009;85:40–52.
Toh WH, Tan JZ, Zulkefli KL, Houghton FJ, Gleeson PA. Amyloid precursor protein traffics from the Golgi directly to early endosomes in an Arl5b- and AP4-dependent pathway. Traffic. 2017;18:159–75.
Acknowledgements
The Alzheimer’s Disease Sequencing Project (ADSP) is comprised of two Alzheimer’s Disease (AD) genetics consortia and three National Human Genome Research Institute (NHGRI) funded Large Scale Sequencing and Analysis Centers (LSAC). The two AD genetics consortia are the Alzheimer’s Disease Genetics Consortium (ADGC) funded by NIA (U01 AG032984), and the Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) funded by NIA (R01 AG033193), the National Heart, Lung, and Blood Institute (NHLBI), other National Institute of Health (NIH) institutes and other foreign governmental and non-governmental organizations. The Discovery Phase analysis of sequence data is supported through UF1AG047133 (to Drs. Farrer, Haines, Mayeux, Pericak-Vance, and Schellenberg); U01AG049505 to Dr. Seshadri; U01AG049506 to Dr. Boerwinkle; U01AG049507 to Dr. Wijsman; and U01AG049508 to Dr. Goate and the Discovery Extension Phase analysis is supported through U01AG052411 to Dr. Goate, U01AG052410 to Dr. Pericak-Vance and U01 AG052409 to Drs. Seshadri and Fornage. Data generation and harmonization in the Follow-up Phases is supported by U54AG052427 to Drs. Schellenberg and Wang. The ADGC cohorts include: Adult Changes in Thought (ACT supported by NIA grant U01AG006781 to Drs. Larson and Crane), the Alzheimer’s Disease Centers (ADC), the Chicago Health and Aging Project (CHAP), the Memory and Aging Project (MAP), Mayo Clinic (MAYO), Mayo Parkinson’s Disease controls, University of Miami, the Multi-Institutional Research in Alzheimer’s Genetic Epidemiology Study (MIRAGE), the National Cell Repository for Alzheimer’s Disease (NCRAD), the National Institute on Aging Late Onset Alzheimer's Disease Family Study (NIA-LOAD), the Religious Orders Study (ROS), the Texas Alzheimer’s Research and Care Consortium (TARC), Vanderbilt University/Case Western Reserve University (VAN/CWRU), the Washington Heights-Inwood Columbia Aging Project (WHICAP supported by NIA grant RF1AG054023 to Dr. Mayeux) and the Washington University Sequencing Project (WUSP), the Columbia University Hispanic- Estudio Familiar de Influencia Genetica de Alzheimer (EFIGA supported by NIA grant RF1AG015473 to Dr. Mayeux), the University of Toronto (UT), and Genetic Differences (GD). Analysis of ADGC cohorts us supported by NIA grants R01AG048927 and RF1AG057519 to Dr. Farrer. Efforts of ADGC investigators were also supported by grants from the NIA (R03AG054936) and National Library of Medicine (R01LM012535). The CHARGE cohorts are supported in part by National Heart, Lung, and Blood Institute (NHLBI) infrastructure grant R01HL105756 (Psaty), RC2HL102419 (Boerwinkle) and the neurology working group is supported by the National Institute on Aging (NIA) R01 grant AG033193. The CHARGE cohorts participating in the ADSP include the following: Austrian Stroke Prevention Study (ASPS), ASPS-Family study, and the Prospective Dementia Registry-Austria (ASPS/PRODEM-Aus), the Atherosclerosis Risk in Communities (ARIC) Study, the Cardiovascular Health Study (CHS), the Erasmus Rucphen Family Study (ERF), the Framingham Heart Study (FHS), and the Rotterdam Study (RS). ASPS is funded by the Austrian Science Fond (FWF) grant number P20545-P05 and P13180 and the Medical University of Graz. The ASPS-Fam is funded by the Austrian Science Fund (FWF) project I904),the EU Joint Programme - Neurodegenerative Disease Research (JPND) in frame of the BRIDGET project (Austria, Ministry of Science) and the Medical University of Graz and the Steiermärkische Krankenanstalten Gesellschaft. PRODEM-Austria is supported by the Austrian Research Promotion agency (FFG) (Project No. 827462) and by the Austrian National Bank (Anniversary Fund, project 15435. ARIC research is carried out as a collaborativestudysupportedbyNHLBIcontracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data in ARIC is collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, NINDS, NIA and NIDCD), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI. CHS research was supported by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grants U01HL080295 and U01HL130114 from the NHLBI with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629, R01AG15928, and R01AG20098 from the NIA. FHS research is supported by NHLBI contracts N01-HC-25195 and HHSN268201500001I. This study was also supported by additional grants from the NIA (R01s AG054076, AG049607 and AG033040 and NINDS (R01NS017950). The ERF study as a part of EUROSPAN (European Special Populations Research Network) was supported by European Commission FP6 STRP grant number 018947 (LSHG-CT-2006-01947) and also received funding from the European Community's Seventh Framework Programme (FP7/2007-2013)/grant agreement HEALTH-F4-2007-201413 by the European Commission under the programme "Quality of Life and Management of the Living Resources" of 5th Framework Programme (no. QLG2-CT-2002-01254). High-throughput analysis of the ERF data was supported by a joint grant from the Netherlands Organization for Scientific Research and the Russian Foundation for Basic Research (NWO-RFBR 047.017.043). The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, the Netherlands Organization for Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the municipality of Rotterdam. Genetic data sets are also supported by the Netherlands Organization of Scientific Research NWO Investments (175.010.2005.011, 911-03-012), the Genetic Laboratory of the Department of Internal Medicine, Erasmus MC, the Research Institute for Diseases in the Elderly (014-93-015; RIDE2), and the Netherlands Genomics Initiative (NGI)/Netherlands Organization for Scientific Research (NWO) Netherlands Consortium for Healthy Aging (NCHA), project 050-060-810. All studies are grateful to their participants, faculty and staff. The content of these manuscripts is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Department of Health and Human Services. The ADES-FR study was funded by grants from the Clinical Research Hospital Program from the French Ministry of Health (GMAJ, PHRC, 2008/067), the CNR-MAJ, the JPND PERADES, the GENMED labex (LABEX GENMED ANR-10-LABX-0013), and the FP7 AgedBrainSysBio. Whole exome sequencing in the 3C-Dijon study was funded by the Fondation Leducq. This work was supported by the France Génomique National infrastructure, funded as part of the Investissements d’Avenir program managed by the Agence Nationale pour la Recherche (ANR-10-INBS-09), the Centre National de Recherche en Génomique Humaine, the National Foundation forAlzheimer's disease and related disorders, the Institut Pasteur de Lille, Inserm, the Lille Métropole Communauté Urbaine council, and the French government's LABEX (laboratory of excellence program investment for the future) DISTALZ grant (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer's disease). The 3C Study supports are listed on the Study Website (www.three-city-study.com). The FinnAD Study at the University of Tampere was supported by The Academy of Finland: grants 286284 (T.L), Competitive State Research Financing of the Expert Responsibility area of Tampere University Hospitals (grant X51001); Juho Vainio Foundation; Paavo Nurmi Foundation; Finnish Foundation for Cardiovascular Research; Finnish Cultural Foundation; Tampere Tuberculosis Foundation; Yrjö Jahnsson Foundation; Signe and Ane Gyllenberg Foundation; and Diabetes Research Foundation of Finnish Diabetes Association. The FinnAD Study at the University of Eastern Finland was supported by the Academy of Finland grant 307866, the Sigrid Jusélius Foundation, and the Strategic Neuroscience Funding of the University of Eastern Finland. The three LSACs are: the Human Genome Sequencing Center at the Baylor College of Medicine (U54 HG003273), the Broad Institute Genome Center (U54HG003067), and the Washington University Genome Institute (U54HG003079).
Biological samples and associated phenotypic data used in primary data analyses were stored at Study Investigator institutions, and at the National Cell Repository for Alzheimer’s Disease (NCRAD, U24AG021886) at Indiana University funded by NIA. Associated Phenotypic Data used in primary and secondary data analyses were provided by Study Investigators, the NIA funded Alzheimer’s Disease Centers (ADCs), and the National Alzheimer’s Coordinating Center (NACC, U01AG016976) and the National Institute on Aging Genetics of Alzheimer’s Disease Data Storage Site (NIAGADS, U24AG041689) at the University of Pennsylvania, funded by NIA, and at the Database for Genotypes and Phenotypes (dbGaP) funded by NIH. This research was supported in part by the Intramural Research Program of the National Institutes of health, National Library of Medicine. Contributors to the Genetic Analysis Data included Study Investigators on projects that were individually funded by NIA, and other NIH institutes, and by private U.S. organizations, or foreign governmental or nongovernmental organizations. We also acknowledge the investigators who assembled and characterized participants of cohorts included in this study:
Adult Changes in Thought: James D. Bowen, Paul K. Crane, Gail P. Jarvik, C. Dirk Keene, Eric B. Larson, W. William Lee, Wayne C. McCormick, Susan M. McCurry, Shubhabrata Mukherjee, Katie Rose Richmire Atherosclerosis Risk in Communities Study: Rebecca Gottesman, David Knopman, Thomas H. Mosley, B. Gwen Windham,
Austrian Stroke Prevention Study: Thomas Benke, Peter Dal-Bianco, Edith Hofer, Gerhard Ransmayr, Yasaman Saba
Cardiovascular Health Study: James T. Becker, Joshua C. Bis, Annette L. Fitzpatrick, M. Ilyas Kamboh, Lewis H. Kuller, WT Longstreth, Jr, Oscar L. Lopez, Bruce M. Psaty, Jerome I. Rotter,
Chicago Health and Aging Project: Philip L. De Jager, Denis A. Evans
Erasmus Rucphen Family Study: Hieab H. Adams, Hata Comic, Albert Hofman, Peter J. Koudstaal, Fernando Rivadeneira, Andre G. Uitterlinden, Dina Voijnovic
Estudio Familiar de la Influencia Genetica en Alzheimer: Sandra Barral, Rafael Lantigua, Richard Mayeux, Martin Medrano, Dolly Reyes-Dumeyer, Badri Vardarajan
Framingham Heart Study: Alexa S. Beiser, Vincent Chouraki, Jayanadra J. Himali, Charles C. White
Genetic Differences: Duane Beekly, James Bowen, Walter A. Kukull, Eric B. Larson, Wayne McCormick, Gerard D. Schellenberg, Linda Teri
Mayo Clinic: Minerva M. Carrasquillo, Dennis W. Dickson, Nilufer Ertekin-Taner, Neill R. Graff-Radford, Joseph E. Parisi, Ronald C. Petersen, Steven G. Younkin
Mayo PD: Gary W. Beecham, Dennis W. Dickson, Ranjan Duara, Nilufer Ertekin-Taner, Tatiana M. Foroud, Neill R. Graff-Radford, Richard B. Lipton, Joseph E. Parisi, Ronald C. Petersen, Bill Scott, Jeffery M. Vance
Memory and Aging Project: David A. Bennett, Philip L. De Jager
Multi-Institutional Research in Alzheimer's Genetic Epidemiology Study: Sanford Auerbach, Helan Chui, Jaeyoon Chung, L. Adrienne Cupples, Charles DeCarli, Ranjan Duara, Martin Farlow, Lindsay A. Farrer, Robert Friedland, Rodney C.P. Go, Robert C. Green, Patrick Griffith, John Growdon, Gyungah R. Jun, Walter Kukull, Alexander Kurz, Mark Logue, Kathryn L. Lunetta, Thomas Obisesan, Helen Petrovitch, Marwan Sabbagh, A. Dessa Sadovnick, Magda Tsolaki
National Cell Repository for Alzheimer's Disease: Kelley M. Faber, Tatiana M. Foroud
National Institute on Aging (NIA) Late Onset Alzheimer's Disease Family Study: David A. Bennett, Sarah Bertelsen, Thomas D. Bird, Bradley F. Boeve, Carlos Cruchaga, Kelley Faber, Martin Farlow, Tatiana M Foroud, Alison M Goate, Neill R. Graff-Radford, Richard Mayeux, Ruth Ottman, Dolly Reyes-Dumeyer, Roger Rosenberg, Daniel Schaid, Robert A Sweet, Giuseppe Tosto, Debby Tsuang, Badri Vardarajan
NIA Alzheimer Disease Centers: Erin Abner, Marilyn S. Albert, Roger L. Albin, Liana G. Apostolova, Sanjay Asthana, Craig S. Atwood, Lisa L. Barnes, Thomas G. Beach, David A. Bennett, Eileen H. Bigio, Thomas D. Bird, Deborah Blacker, Adam Boxer, James B. Brewer, James R. Burke, Jeffrey M. Burns, Joseph D. Buxbaum, Nigel J. Cairns, Chuanhai Cao, Cynthia M. Carlsson, Richard J. Caselli, Helena C. Chui, Carlos Cruchaga, Mony de Leon, Charles DeCarli, Malcolm Dick, Dennis W. Dickson, Nilufer Ertekin-Taner, David W. Fardo, Martin R. Farlow, Lindsay A. Farrer, Steven Ferris, Tatiana M. Foroud, Matthew P. Frosch, Douglas R. Galasko, Marla Gearing, David S. Geldmacher, Daniel H. Geschwind, Bernardino Ghetti, Carey Gleason, Alison M. Goate, Teresa Gomez-Isla, Thomas Grabowski, Neill R. Graff-Radford, John H. Growdon, Lawrence S. Honig, Ryan M. Huebinger, Matthew J. Huentelman, Christine M. Hulette, Bradley T. Hyman, Suman Jayadev, Lee-Way Jin, Sterling Johnson, M. Ilyas Kamboh, Anna Karydas, Jeffrey A. Kaye, C. Dirk Keene, Ronald Kim, Neil W Kowall, Joel H. Kramer, Frank M. LaFerla, James J. Lah, Allan I. Levey, Ge Li, Andrew P. Lieberman, Oscar L. Lopez, Constantine G. Lyketsos, Daniel C. Marson, Ann C. McKee, Marsel Mesulam, Jesse Mez, Bruce L. Miller, Carol A. Miller, Abhay Moghekar, John C. Morris, John M. Olichney, Joseph E. Parisi, Henry L. Paulson, Elaine Peskind, Ronald C. Petersen, Aimee Pierce, Wayne W. Poon, Luigi Puglielli, Joseph F. Quinn, Ashok Raj, Murray Raskind, Eric M. Reiman, Barry Reisberg, Robert A. Rissman, Erik D. Roberson, Howard J. Rosen, Roger N. Rosenberg, Martin Sadowski, Mark A. Sager, David P. Salmon, Mary Sano, Andrew J. Saykin, Julie A. Schneider, Lon S. Schneider, William W. Seeley, Scott Small, Amanda G. Smith, Robert A. Stern, Russell H. Swerdlow, Rudolph E. Tanzi, Sarah E Tomaszewski Farias, John Q. Trojanowski, Juan C. Troncoso, Debby W. Tsuang, Vivianna M. Van Deerlin, Linda J. Van Eldik, Harry V. Vinters, Jean Paul Vonsattel, Jen Chyong Wang, Sandra Weintraub, Kathleen A. Welsh-Bohmer, Shawn Westaway, Thomas S. Wingo, Thomas Wisniewski, David A. Wolk, Randall L. Woltjer, Steven G. Younkin, Lei Yu, Chang-En Yu
Religious Orders Study: David A. Bennett, Philip L. De Jager
Rotterdam Study: Kamran Ikram, Frank J Wolters
Texas Alzheimer's Research and Care Consortium: Perrie Adams, Alyssa Aguirre, Lisa Alvarez, Gayle Ayres, Robert C. Barber, John Bertelson, Sarah Brisebois, Scott Chasse, Munro Culum, Eveleen Darby, John C. DeToledo, Thomas J. Fairchild, James R. Hall, John Hart, Michelle Hernandez, Ryan Huebinger, Leigh Johnson, Kim Johnson, Aisha Khaleeq, Janice Knebl, Laura J. Lacritz, Douglas Mains, Paul Massman, Trung Nguyen, Sid O’Bryant, Marcia Ory, Raymond Palmer, Valory Pavlik, David Paydarfar, Victoria Perez, Marsha Polk, Mary Quiceno, Joan S. Reisch, Monica Rodriguear, Roger Rosenberg, Donald R. Royall, Janet Smith, Alan Stevens, Jeffrey L. Tilson, April Wiechmann, Kirk C. Wilhelmsen, Benjamin Williams, Henrick Wilms, Martin Woon
University of Miami: Larry D Adams, Gary W. Beecham, Regina M Carney, Katrina Celis, Michael L Cuccaro, Kara L. Hamilton-Nelson, James Jaworski, Brian W. Kunkle, Eden R. Martin, Margaret A. Pericak-Vance, Farid Rajabli, Michael Schmidt, Jeffery M Vance
University of Toronto: Ekaterina Rogaeva, Peter St. George-Hyslop
University of Washington Families: Thomas D. Bird, Olena Korvatska, Wendy Raskind, Chang-En Yu
Vanderbilt University: John H. Dougherty, Harry E. Gwirtsman, Jonathan L. Haines
Washington Heights-Inwood Columbia Aging Project: Adam Brickman, Rafael Lantigua, Jennifer Manly, Richard Mayeux, Christiane Reitz, Nicole Schupf, Yaakov Stern, Giuseppe Tosto, Badri Vardarajan
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Celine Bellenguez received personal fees from Genoscrenn unrelated to current study. HS reports grants from Austrian Science Fond (FWF) and Österreichische Nationalbank Anniversary Fund during the conduct of the study. AMG received grants from NIA, Genentech, Pfizer, and Astra Zeneca as well as personal fees from Finnegan HC, Cognition Therapeutics, Dickstein Shapiro, Genentech, and Amgen, all unrelated to the current study. In addition, AMG has a patent (US20070258898) issued, and a patent with royalties paid by Taconic. J-FD received grants and personal fees from IPSEN and Novartis, and personal fees from Newron. MAP-V received personal fees from Athena Neurosciences unrelated to current study. The other authors declare no conflict of interest.
Additional information
Alzheimer’s Disease Sequencing Project members are listed below the Acknowledgement
Electronic supplementary material
Alzheimer’s disease sequencing project members
Alzheimer’s disease sequencing project members
Baylor College of Medicine: Michelle Bellair, Huyen Dinh, Harsha Doddapeneni, Shannon Dugan-Perez, Adam English, Richard A. Gibbs, Yi Han, Jianhong Hu, Joy Jayaseelan, Divya Kalra, Ziad Khan, Viktoriya Korchina, Sandra Lee, Yue Liu, Xiuping Liu, Donna Muzny, Waleed Nasser, William Salerno, Jireh Santibanez, Evette Skinner, Simon White, Kim Worley, Yiming Zhu
Boston University: Alexa Beiser, Yuning Chen, Jaeyoon Chung, L. Adrienne Cupples, Anita DeStefano, Josee Dupuis, John Farrell, Lindsay Farrer, Daniel Lancour, Honghuang Lin, Ching Ti Liu, Kathy Lunetta, Yiyi Ma, Devanshi Patel, Chloe Sarnowski, Claudia Satizabal, Sudha Seshadri, Fangui Jenny Sun, Xiaoling Zhang
Broad Institute: Seung Hoan Choi, Eric Banks, Stacey Gabriel, Namrata Gupta
Case Western Reserve University: William Bush, Mariusz Butkiewicz, Jonathan Haines, Sandra Smieszek, Yeunjoo Song
Columbia University: Sandra Barral, Phillip L De Jager, Richard Mayeux, Christiane Reitz, Dolly Reyes, Giuseppe Tosto, Badri Vardarajan
Erasmus Medical University: Shahzad Amad, Najaf Amin, M Afran Ikram, Sven van der Lee, Cornelia van Duijn, Ashley Vanderspek
Medical University Graz:Helena Schmidt, Reinhold Schmidt
Mount Sinai School of Medicine:Alison Goate, Manav Kapoor, Edoardo Marcora, Alan Renton
Indiana University: Kelley Faber, Tatiana Foroud
National Center Biotechnology Information:Michael Feolo, Adam Stine
National Institute on Aging: Lenore J. Launer
Rush University: David A Bennett
Stanford University: Li Charlie Xia
University of Miami: Gary Beecham, Kara Hamilton-Nelson, James Jaworski, Brian Kunkle, Eden Martin, Margaret Pericak-Vance, Farid Rajabli, Michael Schmidt
University of Mississippi: Thomas H. Mosley
University of Pennsylvania: Laura Cantwell, Micah Childress, Yi-Fan Chou, Rebecca Cweibel, Prabhakaran Gangadharan, Amanda Kuzma, Yuk Yee Leung, Han-Jen Lin, John Malamon, Elisabeth Mlynarski, Adam Naj, Liming Qu, Gerard Schellenberg, Otto Valladares, Li-San Wang, Weixin Wang, Nancy Zhang
University of Texas Houston: Jennifer E. Below, Eric Boerwinkle, Jan Bressler, Myriam Fornage, Xueqiu Jian, Xiaoming Liu
University of Washington: Joshua C. Bis, Elizabeth Blue, Lisa Brown, Tyler Day, Michael Dorschner, Andrea R Horimoto, Rafael Nafikov, Alejandro Q Nato Jr., Pat Navas, Hiep Nguyen, Bruce Psaty, Kenneth Rice, Mohamad Saad, Harkirat Sohi, Timothy Thornton, Debby Tsuang, Bowen Wang, Ellen Wijsman, Daniela Witten
Washington University: Lucinda Antonacci-Fulton, Elizabeth Appelbaum, Carlos Cruchaga, Robert S. Fulton, Daniel C. Koboldt, David E. Larson, Jason Waligorski, Richard K. Wilson
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bis, J.C., Jian, X., Kunkle, B.W. et al. Whole exome sequencing study identifies novel rare and common Alzheimer’s-Associated variants involved in immune response and transcriptional regulation. Mol Psychiatry 25, 1859–1875 (2020). https://doi.org/10.1038/s41380-018-0112-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0112-7
This article is cited by
-
Multi-omics and pathway analyses of genome-wide associations implicate regulation and immunity in verbal declarative memory performance
Alzheimer's Research & Therapy (2024)
-
Human whole-exome genotype data for Alzheimer’s disease
Nature Communications (2024)
-
The neuroimmune axis of Alzheimer’s disease
Genome Medicine (2023)
-
Convergent transcriptomic and genomic evidence supporting a dysregulation of CXCL16 and CCL5 in Alzheimer’s disease
Alzheimer's Research & Therapy (2023)
-
Neuroinflammation in Alzheimer’s disease: microglial signature and their relevance to disease
Inflammation and Regeneration (2023)