Abstract
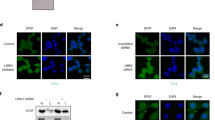
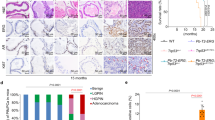
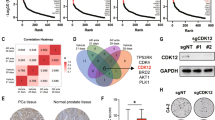
SCFSkp2/Cks1 ubiquitinates Thr187-phosphorylated p27 for degradation. Overexpression of Skp2 coupled with underexpression of p27 are frequent characteristics of cancer cells. When the role of SCFSkp2/Cks1-mediated p27 ubiquitination in cancer was specifically tested by p27 Thr187-to-Ala knockin (p27T187A KI), it was found dispensable for KrasG12D-induced lung tumorigenesis but essential for Rb1-deficient pituitary tumorigenesis. Here we identify pRb and p53 doubly deficient (DKO) prostate tumorigenesis as a context in which p27 ubiquitination by SCFSkp2/Cks1 is required for p27 downregulation. p27 protein accumulated in prostate when p27T187A KI mice underwent DKO prostate tumorigenesis. p27T187A KI or Skp2 knockdown (KD) induced similar degrees of p27 protein accumulation in DKO prostate cells, and Skp2 KD did not further increase p27 protein in DKO prostate cells that contained p27T187A KI (AADKO prostate cells). p27T187A KI activated an E2F1-p73-apoptosis axis in DKO prostate tumorigenesis, slowed disease progression and significantly extended survival. Querying co-occurrence relationships among RB1, TP53, PTEN, NKX3-1 and MYC in TCGA of prostate cancer identified co-inactivation of RB1 and TP53 as the only statistically significant co-occurrences in metastatic castration-resistant prostate cancer (mCRPC). Together, our study identifies Skp2/Cks1 pocket inhibitors as potential therapeutics for mCRPC. Procedures for establishing mCRPC organoid cultures from contemporary patients were recently established. An Skp2/Cks1 pocket inhibitor preferentially collapsed DKO prostate tumor organoids over AADKO organoids, which spontaneously disintegrated over time when DKO prostate tumor organoids grew larger, setting the stage to translate mouse model findings to precision medicine in the clinic on the organoid platform.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Starostina NG, Kipreos ET . Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol 2012; 22: 33–41.
Vlach J, Hennecke S, Amati B . Phosphorylation-dependent degradation of the cyclin-dependent kinase inhibitor p27. EMBO J 1997; 16: 5334–5344.
Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A et al. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev 1999; 13: 1181–1189.
Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M et al. The cell-cycle regulatory protein Cks1 is required for SCFSkp2-mediated ubiquitinylation of p27. Nat Cell Biol 2001; 3: 321–324.
Spruck C, Strohmaier H, Watson M, Smith APL, Ryan A, Krek W et al. A CDK-independent function of mammalian Cks1: targeting of SCFSkp2 to the CDK inhibitor p27Kip1. Mol Cell 2001; 7: 639–650.
Hao B, Zheng N, Schulman BA, Wu G, Miller JJ, Pagano M et al. Structural basis of the Cks1-dependent recognition of p27(Kip1) by the SCF(Skp2) ubiquitin ligase. Mol Cell 2005; 20: 9–19.
Malek NP, Sundberg H, McGrew S, Nakayama K, Kyriakidis TR, Roberts JM . A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 2001; 413: 323–327.
Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ . The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 1998; 396: 177–180.
Chu IM, Hengst L, Slingerland JM . The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 2008; 8: 253–267.
Timmerbeul I, Garrett-Engele CM, Kossatz U, Chen X, Firpo E, Grunwald V et al. Testing the importance of p27 degradation by the SCFskp2 pathway in murine models of lung and colon cancer. Proc Natl Acad Sci USA 2006; 103: 14009–14014.
Park MS, Rosai J, Nguyen HT, Capodieci P, Cordon-Cardo C, Koff A . p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc Natl Acad Sci USA 1999; 96: 6382–6387.
Zhao H, Bauzon F, Bi E, Yu JJ, Fu H, Lu Z et al. Substituting threonine187 with alanine in p27Kip1 prevents pituitary tumorigenesis by two-hit loss of Rb1 and enhances humoral immunity in old age. J Biol Chem 2015; 290: 5797–5809.
Wu L, Grigoryan AV, Li Y, Hao B, Pagano M, Cardozo TJ . Specific small molecule inhibitors of Skp2-mediated p27 degradation. Chem Biology 2012; 19: 1515–1524.
Skaar JR, Pagan JK, Pagano M . SCF ubiquitin ligase-targeted therapies. Nat Rev Drug Disc 2014; 13: 889–903.
Wang H, Bauzon F, Ji P, Xu X, Sun D, Locker J et al. Skp2 is required for survival of aberrantly proliferating Rb1-deficient cells and for tumorigenesis in Rb1+/- mice. Nat Genet 2010; 42: 83–88.
Zhao H, Bauzon F, Fu H, Lu Z, Cui J, Nakayama K et al. Skp2 deletion unmasks a p27 safeguard that blocks tumorigenesis in the absence of pRb and p53 tumor suppressors. Cancer Cell 2013 11; 24: 645–659.
Lu Z, Bauzon F, Fu H, Cui J, Zhao H, Nakayama K et al. Skp2 suppresses apoptosis in Rb1-deficient tumours by limiting E2F1 activity. Nat Commun 2014; 5: 3463.
Candi E, Agostini M, Melino G, Bernassola F . How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: regulators and effectors. Hum Mutat 2014; 35: 702–714.
Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res 2006; 66: 7889–7898.
Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K et al. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J 2000; 19: 2069–2081.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1.
Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012; 483: 603–607.
Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014; 159: 176–187.
Yang G, Ayala G, De Marzo A, Tian W, Frolov A, Wheeler TM et al. Elevated Skp2 protein expression in human prostate cancer: association with loss of the cyclin-dependent kinase inhibitor p27 and PTEN and with reduced recurrence-free survival. Clin Cancer Res 2002; 8: 3419–3426.
Ji P, Jiang H, Rekhtman K, Bloom J, Ichetovkin M, Pagano M et al. An Rb-Skp2-p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol Cell 2004; 16: 47–58.
Zhang L, Wang C . F-box protein Skp2: a novel transcriptional target of E2F. Oncogene 2005; 25: 2615–2627.
Yung Y, Walker JL, Roberts JM, Assoian RK . A Skp2 autoinduction loop and restriction point control. J Cell Biol 2007; 178: 741–747.
Binne UK, Classon MK, Dick FA, Wei W, Rape M, Kaelin WG Jr et al. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat Cell Biol 2007; 9: 225–232.
Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H et al. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2). Curr Biol 2001; 11: 263–267.
Lin HK, Chen Z, Wang G, Nardella C, Lee SW, Chan CH et al. Skp2 targeting suppresses tumorigenesis by Arf-p53-independent cellular senescence. Nature 2010; 464: 374–379.
Hattori T, Isobe T, Abe K, Kikuchi H, Kitagawa K, Oda T et al. Pirh2 promotes ubiquitin-dependent degradation of the cyclin-dependent kinase inhibitor p27Kip1. Cancer Res 2007; 67: 10789–10795.
Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S et al. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27(Kip1) at G1 phase. Nat Cell Biol 2004; 6: 1229–1235.
Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev 2008; 22: 3121–3134.
Cao X, Xue L, Han L, Ma L, Chen T, Tong T . WW domain-containing E3 ubiquitin protein ligase 1 (WWP1) delays cellular senescence by promoting p27(Kip1) degradation in human diploid fibroblasts. J Biol Chem 2011; 286: 33447–33456.
Bassermann F, Eichner R, Pagano M . The ubiquitin proteasome system - implications for cell cycle control and the targeted treatment of cancer. Biochim Biophys Acta 2014; 1843: 150–162.
Orlowski RZ, Kuhn DJ . Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res 2008; 14: 1649–1657.
Morris MJ, Kelly WK, Slovin S, Ryan C, Eicher C, Heller G et al. A phase II trial of bortezomib and prednisone for castration resistant metastatic prostate cancer. J Urol 2007; 178: 2378–2383.
Jin Y, Lee H, Zeng SX, Dai MS, Lu H . MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J 2003; 22: 6365–6377.
Amador V, Ge S, Santamaria PG, Guardavaccaro D, Pagano M . APC/C(Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell 2007; 27: 462–473.
Havens CG, Walter JC . Docking of a specialized PIP box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell 2009; 35: 93–104.
Starostina NG, Simpliciano JM, McGuirk MA, Kipreos ET . CRL2(LRR-1) targets a CDK inhibitor for cell cycle control in C. elegans and actin-based motility regulation in human cells. Dev Cell 2010; 19: 753–764.
Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell 2013; 154: 556–568.
Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF . Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 1978; 21: 274–281.
Gillet JP, Varma S, Gottesman MM . The clinical relevance of cancer cell lines. J Natl Cancer Inst 2013; 105: 452–458.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N . Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 2013; 4: 2126.
Sato T, Clevers H . SnapShot: growing organoids from stem cells. Cell 2015; 161: 1700–e1.
van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 2015; 161: 933–945.
Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014; 159: 163–175.
Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nat Cell Biol 2014; 16: 951–961.
Wu X, Wu J, Huang J, Powell WC, Zhang J, Matusik RJ et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 2001; 101: 61–69.
Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T . Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature 2003; 424: 223–228.
Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A . Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet 2001; 29: 418–425.
Sun D, Melegari M, Sridhar S, Rogler CE, Zhu L . A multi-miRNA hairpin method that improves gene knockdown efficiency and provides linked multi-gene knockdown. BioTechniques 2006; 41: 59–63.
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18: 11–22.
Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012; 44: 685–689.
TCGA. The molecular taxonomy of primary prostate cancer. Cell 2015; 163: 1011–1025.
Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487: 239–243.
Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161: 1215–1228.
Acknowledgements
This work was supported by NIH grants RO1CA127901and RO1CA131421 (LZ), Albert Einstein Comprehensive Cancer Research Center (5P30CA13330) and Liver Research Center (5P30DK061153) provided core facility support. HZ was a recipient of DOD PCRP Postdoctoral Fellowship (PC121837), and LZ was a Irma T Hirschl Career Scientist Award recipient. We thank Dr James Roberts for providing the p27T187A KI mice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, H., Lu, Z., Bauzon, F. et al. p27T187A knockin identifies Skp2/Cks1 pocket inhibitors for advanced prostate cancer. Oncogene 36, 60–70 (2017). https://doi.org/10.1038/onc.2016.175
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.175
This article is cited by
-
O-GlcNAcylation of E3 ubiquitin ligase SKP2 promotes hepatocellular carcinoma proliferation
Oncogene (2024)
-
Targeted inhibition of SCFSKP2 confers anti-tumor activities resulting in a survival benefit in osteosarcoma
Oncogene (2024)
-
Targeting the untargetable: RB1-deficient tumours are vulnerable to Skp2 ubiquitin ligase inhibition
British Journal of Cancer (2022)
-
CHPF promotes gastric cancer tumorigenesis through the activation of E2F1
Cell Death & Disease (2021)
-
The heavy chain of 4F2 antigen promote prostate cancer progression via SKP-2
Scientific Reports (2021)