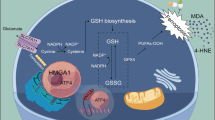
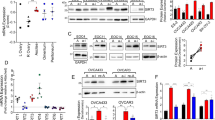
Abstract
Epithelial–mesenchymal transition (EMT) promotes cancer cell invasion, metastasis and treatment failure. EMT may be activated in cancer cells by reactive oxygen species (ROS). EMT may promote conversion of a subset of cancer cells from a CD44low-CD24high (CD44L) epithelial phenotype to a CD44high-CD24−/low (CD44H) mesenchymal phenotype, the latter associated with increased malignant properties of cancer cells. ROS are required for cells undergoing EMT, although excessive ROS may induce cell death or senescence; however, little is known as to how cellular antioxidant capabilities may be regulated during EMT. Mitochondrial superoxide dismutase 2 (SOD2) is frequently overexpressed in oral and esophageal cancers. Here, we investigate mechanisms of SOD2 transcriptional regulation in EMT, as well as the functional role of this antioxidant in EMT. Using well-characterized genetically engineered oral and esophageal human epithelial cell lines coupled with RNA interference and flow cytometric approaches, we find that transforming growth factor (TGF)-β stimulates EMT, resulting in conversion of CD44L to CD44H cells, the latter of which display SOD2 upregulation. SOD2 induction in transformed keratinocytes was concurrent with suppression of TGF-β-mediated induction of both ROS and senescence. SOD2 gene expression appeared to be transcriptionally regulated by NF-κB and ZEB2, but not ZEB1. Moreover, SOD2-mediated antioxidant activity may restrict conversion of CD44L cells to CD44H cells at the early stages of EMT. These data provide novel mechanistic insights into the dynamic expression of SOD2 during EMT. In addition, we delineate a functional role for SOD2 in EMT via the influence of this antioxidant upon distinct CD44L and CD44H subsets of cancer cells that have been implicated in oral and esophageal tumor biology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Nieto MA . The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol 2011; 27: 347–376.
Kalluri R, Weinberg RA . The basics of epithelial-mesenchymal transition. J Clin Invest 2009; 119: 1420–1428.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
De Craene B, Berx G . Regulatory networks defining EMT during cancer initiation and progression. Nat Rev Cancer 2013; 13: 97–110.
Giannoni E, Parri M, Chiarugi P . EMT and oxidative stress: a bidirectional interplay affecting tumor malignancy. Antioxid Redox Signal 2012; 16: 1248–1263.
Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997; 275: 1649–1652.
Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 2008; 14: 79–89.
Zoller M . CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011; 11: 254–267.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF . Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 2003; 100: 3983–3988.
Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA 2007; 104: 973–978.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008; 133: 704–715.
Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A . Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One 2008; 3: e2888.
Sena LA, Chandel NS . Physiological roles of mitochondrial reactive oxygen species. Mol Cell 2012; 48: 158–167.
Thannickal VJ, Fanburg BL . Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000; 279: L1005–L1028.
Lambert AJ, Brand MD . Reactive oxygen species production by mitochondria. Methods Mol Biol 2009; 554: 165–181.
Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 2005; 436: 123–127.
Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q et al. Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1. Am J Physiol Lung Cell Mol Physiol 2009; 297: L1120–L1130.
Kagawa S, Natsuizaka M, Whelan KA, Facompre N, Naganuma S, Ohashi S et al. Cellular senescence checkpoint function determines differential Notch1-dependent oncogenic and tumor-suppressor activities. Oncogene 2014, e-pub ahead of print 16 June 2014; doi:10.1038/onc.2014.169.
Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G . TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 2005; 24: 1895–1903.
Ohashi S, Natsuizaka M, Wong GS, Michaylira CZ, Grugan KD, Stairs DB et al. Epidermal growth factor receptor and mutant p53 expand an esophageal cellular subpopulation capable of epithelial-to-mesenchymal transition through ZEB transcription factors. Cancer Res 2010; 70: 4174–4184.
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P . Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1374.
Zelko IN, Mariani TJ, Folz RJ . Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med 2002; 33: 337–349.
Dhar SK St, Clair DK . Manganese superoxide dismutase regulation and cancer. Free Radic Biol Med 2012; 52: 2209–2222.
Connor KM, Hempel N, Nelson KK, Dabiri G, Gamarra A, Belarmino J et al. Manganese superoxide dismutase enhances the invasive and migratory activity of tumor cells. Cancer Res 2007; 67: 10260–10267.
Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest 2004; 114: 569–581.
Thiery JP, Acloque H, Huang RY, Nieto MA . Epithelial-mesenchymal transitions in development and disease. Cell 2009; 139: 871–890.
Lamouille S, Xu J, Derynck R . Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014; 15: 178–196.
Chaudhuri L, Nicholson AM, Kalen AL, Goswami PC . Preferential selection of MnSOD transcripts in proliferating normal and cancer cells. Oncogene 2012; 31: 1207–1216.
Xu Y, Kiningham KK, Devalaraja MN, Yeh CC, Majima H, Kasarskis EJ et al. An intronic NF-kappaB element is essential for induction of the human manganese superoxide dismutase gene by tumor necrosis factor-alpha and interleukin-1beta. DNA Cell Biol 1999; 18: 709–722.
Kim HP, Roe JH, Chock PB, Yim MB . Transcriptional activation of the human manganese superoxide dismutase gene mediated by tetradecanoylphorbol acetate. J Biol Chem 1999; 274: 37455–37460.
Yeh CC, Wan XS, St Clair DK . Transcriptional regulation of the 5' proximal promoter of the human manganese superoxide dismutase gene. DNA Cell Biol 1998; 17: 921–930.
Ohashi S, Natsuizaka M, Naganuma S, Kagawa S, Kimura S, Itoh H et al. A NOTCH3-mediated squamous cell differentiation program limits expansion of EMT-competent cells that express the ZEB transcription factors. Cancer Res 2011; 71: 6836–6847.
Ovcharenko I, Nobrega MA, Loots GG, Stubbs L . ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res 2004; 32: W280–W286.
Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol 2005; 16: 667–675.
Lin CH, Hung PH, Chen YJ . CD44 is associated with the aggressive phenotype of nasopharyngeal carcinoma through redox regulation. Int J Mol Sci 2013; 14: 13266–13281.
Gammon L, Biddle A, Heywood HK, Johannessen AC, Mackenzie IC . Sub-sets of cancer stem cells differ intrinsically in their patterns of oxygen metabolism. PLoS One 2013; 8: e62493.
Natsuizaka M, Kinugasa H, Kagawa S, Whelan KA, Naganuma S, Subramanian H et al. IGFBP3 promotes esophageal cancer growth by suppressing oxidative stress in hypoxic tumor microenvironment. Am J Cancer Res 2014; 4: 29–41.
Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 2011; 19: 387–400.
Dhar SK, Tangpong J, Chaiswing L, Oberley TD, St Clair DK . Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res 2011; 71: 6684–6695.
Guha M, Srinivasan S, Ruthel G, Kashina AK, Carstens RP, Mendoza A et al. Mitochondrial retrograde signaling induces epithelial-mesenchymal transition and generates breast cancer stem cells. Oncogene 2013; 33: 5238–5250.
Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H . NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene 2007; 26: 711–724.
Nam EH, Lee Y, Zhao XF, Park YK, Lee JW, Kim S . ZEB2-Sp1 cooperation induces invasion by upregulating cadherin-11 and integrin alpha5 expression. Carcinogenesis 2014; 35: 302–314.
Nam EH, Lee Y, Park YK, Lee JW, Kim S . ZEB2 upregulates integrin alpha5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis 2012; 33: 563–571.
Chen PM, Wu TC, Wang YC, Cheng YW, Sheu GT, Chen CY et al. Activation of NF-kappaB by SOD2 promotes the aggressiveness of lung adenocarcinoma by modulating NKX2-1-mediated IKKbeta expression. Carcinogenesis 2013; 34: 2655–2663.
Meng X, Wu J, Pan C, Wang H, Ying X, Zhou Y et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology 2013; 145: 426–436.e421–426.
Ji G, Lv K, Chen H, Wang T, Wang Y, Zhao D et al. MiR-146a regulates SOD2 expression in H2O2 stimulated PC12 cells. PLoS One 2013; 8: e69351.
Bai XY, Ma Y, Ding R, Fu B, Shi S, Chen XM . miR-335 and miR-34a Promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol 2011; 22: 1252–1261.
Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR et al. MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: a novel role of miR-382. Nucleic Acids Res 2010; 38: 8338–8347.
Liu X, Yu J, Jiang L, Wang A, Shi F, Ye H et al. MicroRNA-222 regulates cell invasion by targeting matrix metalloproteinase 1 (MMP1) and manganese superoxide dismutase 2 (SOD2) in tongue squamous cell carcinoma cell lines. Cancer Genomics Proteomics 2009; 6: 131–139.
Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X et al. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J 2008; 22: 4126–4135.
Ju X, Casimiro MC, Gormley M, Meng H, Jiao X, Katiyar S et al. Identification of a cyclin D1 network in prostate cancer that antagonizes epithelial-mesenchymal restraint. Cancer Res 2014; 74: 508–519.
Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA et al. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol 2000; 20: 1436–1447.
Acknowledgements
We are grateful to the Molecular Pathology & Imaging, Molecular Biology/Gene Expression and Cell Culture Core Facilities of the NIH/NIDDK Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306) and of the NIH P01CA098101. This study was supported in part by NIH Grants P01CA098101 (to HK, KAW, KT, MN, AL, AG, SC, SK, JAD and HN), F32CA174176 (KAW), F30CA175133 (AL), K26RR032714 (HN), R01CA073599 (DKS) as well as Pennsylvania CURE Program Grant (HN), the Edward P. Evans Foundation (DKS), University of Pennsylvania University Research Foundation Award (HN) and University of Pennsylvania, Abramson Cancer Center Pilot Project Grant (HN).
Abbreviations
CSC, cancer stem cell; EMT, epithelial–mesenchymal transition; FACS, fluorescence-activated cell sorting; RNAi, RNA interference; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; TGF-β, transforming growth factor-β
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Kinugasa, H., Whelan, K., Tanaka, K. et al. Mitochondrial SOD2 regulates epithelial–mesenchymal transition and cell populations defined by differential CD44 expression. Oncogene 34, 5229–5239 (2015). https://doi.org/10.1038/onc.2014.449
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.449
This article is cited by
-
ROS production by mitochondria: function or dysfunction?
Oncogene (2024)
-
Harnessing function of EMT in cancer drug resistance: a metastasis regulator determines chemotherapy response
Cancer and Metastasis Reviews (2024)
-
TGF-β signaling in the tumor metabolic microenvironment and targeted therapies
Journal of Hematology & Oncology (2022)
-
TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer
Cellular and Molecular Life Sciences (2020)
-
Epithelial-stromal crosstalk and fibrosis in eosinophilic esophagitis
Journal of Gastroenterology (2019)