Abstract
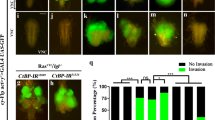
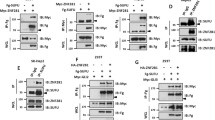
Chromatin remodeling proteins regulate multiple aspects of cell homeostasis, making them ideal candidates for misregulation in transformed cells. Here, we explore Sin3A, a member of the Sin3 family of proteins linked to tumorigenesis that are thought to regulate gene expression through their role as histone deacetylases (HDACs). We identified Drosophila Sin3a as an important mediator of oncogenic Ret receptor in a fly model of Multiple Endocrine Neoplasia Type 2. Reducing Drosophila Sin3a activity led to metastasis-like behavior and, in the presence of Diap1, secondary tumors distant from the site of origin. Genetic and Chip-Seq analyses identified previously undescribed Sin3a targets including genes involved in cell motility and actin dynamics, as well as signaling pathways including Src, Jnk and Rho. A key Sin3a oncogenic target, PP1B, regulates stability of β-Catenin/Armadillo: the outcome is to oppose T-cell factor (TCF) function and Wg/Wnt pathway signaling in both fly and mammalian cancer cells. Reducing Sin3A strongly increased the invasive behavior of A549 human lung adenocarcinoma cells. We show that Sin3A is downregulated in a variety of human tumors and that Src, JNK, RhoA and PP1B/β-Catenin are regulated in a manner analogous to our Drosophila models. Our data suggest that Sin3A influences a specific step of tumorigenesis by regulating a module of genes involved in cell invasion. Tumor progression may commonly rely on such ‘modules of invasion’ under the control of broad transcriptional regulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Lafon-Hughes L, Di Tomaso MV, Mendez-Acuna L, Martinez-Lopez W . Chromatin-remodelling mechanisms in cancer. Mutat Res 2008; 658: 191–214.
Minucci S, Pelicci PG . Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer 2006; 6: 38–51.
Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C . Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science 2010; 330: 1824–1827.
Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 2010; 468: 1105–1109.
Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 2012; 481: 329–334.
Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE . Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell 1997; 89: 341–347.
Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell 1997; 89: 373–380.
Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA . mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev 2005; 19: 1581–1595.
Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL et al. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem 2000; 275: 9461–9467.
Pile LA, Spellman PT, Katzenberger RJ, Wassarman DA . The SIN3 deacetylase complex represses genes encoding mitochondrial proteins: implications for the regulation of energy metabolism. J Biol Chem 2003; 278: 37840–37848.
Alland L, Muhle R, Hou H, Potes J, Chin L, Schreiber-Agus N et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 1997; 387: 49–55.
Rao G, Alland L, Guida P, Schreiber-Agus N, Chen K, Chin L et al. Mouse Sin3A interacts with and can functionally substitute for the amino-terminal repression of the Myc antagonist Mxi1. Oncogene 1996; 12: 1165–1172.
David G, Grandinetti KB, Finnerty PM, Simpson N, Chu GC, Depinho RA . Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci USA. 2008; 105: 4168–4172.
Lai A, Kennedy BK, Barbie DA, Bertos NR, Yang XJ, Theberge MC et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol Cell Biol 2001; 21: 2918–2932.
Murphy M, Ahn J, Walker KK, Hoffman WH, Evans RM, Levine AJ et al. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev 1999; 13: 2490–2501.
Zhang Y, Akinmade D, Hamburger AW . The ErbB3 binding protein Ebp1 interacts with Sin3A to repress E2F1 and AR-mediated transcription. Nucleic Acids Res 2005; 33: 6024–6033.
Hurst DR, Xie Y, Edmonds MD, Welch DR . Multiple forms of BRMS1 are differentially expressed in the MCF10 isogenic breast cancer progression model. Clin Exp Metastasis 2009; 26: 89–96.
Samant RS, Debies MT, Hurst DR, Moore BP, Shevde LA, Welch DR . Suppression of murine mammary carcinoma metastasis by the murine ortholog of breast cancer metastasis suppressor 1 (Brms1). Cancer Lett 2006; 235: 260–265.
Meehan WJ, Samant RS, Hopper JE, Carrozza MJ, Shevde LA, Workman JL et al. Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with retinoblastoma-binding protein 1 (RBP1) and the mSin3 histone deacetylase complex and represses transcription. J Biol Chem 2004; 279: 1562–1569.
Farias EF, Petrie K, Leibovitch B, Murtagh J, Chornet MB, Schenk T et al. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci USA 2010; 107: 11811–11816.
Read RD, Goodfellow PJ, Mardis ER, Novak N, Armstrong JR, Cagan RLA . Drosophila model of multiple endocrine neoplasia type 2. Genetics 2005; 171: 1057–1081.
Plaza-Menacho I, Burzynski GM, de Groot JW, Eggen BJ, Hofstra RM . Current concepts in RET-related genetics, signaling and therapeutics. Trends Genet 2006; 22: 627–636.
Vidal M, Larson DE, Cagan RL . Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell 2006; 10: 33–44.
Ellison-Zelski SJ, Alarid ET . Maximum growth and survival of estrogen receptor-alpha positive breast cancer cells requires the Sin3A transcriptional repressor. Mol Cancer 2010; 9: 263.
Nascimento EM, Cox CL, Macarthur S, Hussain S, Trotter M, Blanco S et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol 2011; 13: 1395–1405.
Moreno-Bueno G, Portillo F, Cano A . Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008; 27: 6958–6969.
Thiery JP . Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002; 2: 442–454.
Negre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U et al. A cis-regulatory map of the Drosophila genome. Nature 2011; 471: 527–531.
van Oevelen C, Wang J, Asp P, Yan Q, Kaelin WG, Kluger Y et al. A role for mammalian Sin3 in permanent gene silencing. Mol Cell 2008; 32: 359–370.
Kouzarides T . Chromatin modifications and their function. Cell 2007; 128: 693–705.
Margueron R, Trojer P, Reinberg D . The key to development: interpreting the histone code? Curr Opin Genet Dev 2005; 15: 163–176.
Cooper JA, Gould KL, Cartwright CA, Hunter T . Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science 1986; 231: 1431–1434.
Iwashita T, Asai N, Murakami H, Matsuyama M, Takahashi M . Identification of tyrosine residues that are essential for transforming activity of the ret proto-oncogene with MEN2A or MEN2B mutation. Oncogene 1996; 12: 481–487.
Songyang Z, Carraway KL, Eck MJ, Harrison SC, Feldman RA, Mohammadi M et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature 1995; 373: 536–539.
Read RD, Bach EA, Cagan RL . Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol Cell Biol 2004; 24: 6676–6689.
Hasegawa T, Enomoto A, Kato T, Kawai K, Miyamoto R, Jijiwa M et al. Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice. Oncogene 2008; 27: 5684–5695.
Uhlirova M, Bohmann D . JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006; 25: 5294–5304.
Teng DH, Perry WL, Hogan JK, Baumgard M, Bell R, Berry S et al. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res 1997; 57: 4177–4182.
Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486: 346–352.
Ahn YH, Yang Y, Gibbons DL, Creighton CJ, Yang F, Wistuba II et al. Map2k4 functions as a tumor suppressor in lung adenocarcinoma and inhibits tumor cell invasion by decreasing peroxisome proliferator-activated receptor gamma2 expression. Mol Cell Biol 2011; 31: 4270–4285.
Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F . A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS ONE 2009; 4: e7709.
Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE et al. Beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell 2011; 20: 741–754.
Parri M, Chiarugi P . Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal 2010; 8: 23.
Duman-Scheel M, Johnston LA, Du W . Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc Natl Acad Sci USA 2004; 101: 3857–3862.
Blauwkamp TA, Chang MV, Cadigan KM . Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J 2008; 27: 1436–1446.
Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F et al. The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res 2009; 69: 1867–1876.
Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM et al. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs 2007; 185: 61–65.
Lilien J, Balsamo J . The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol 2005; 17: 459–465.
Schmalhofer O, Brabletz S, Brabletz T . E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 2009; 28: 151–166.
Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J 2007; 26: 1511–1521.
Pile LA, Schlag EM, Wassarman DA . The SIN3/RPD3 deacetylase complex is essential for G(2) phase cell cycle progression and regulation of SMRTER corepressor levels. Mol Cell Biol 2002; 22: 4965–4976.
Suzuki H, Ouchida M, Yamamoto H, Yano M, Toyooka S, Aoe M et al. Decreased expression of the SIN3A gene, a candidate tumor suppressor located at the prevalent allelic loss region 15q23 in non-small cell lung cancer. Lung Cancer 2008; 59: 24–31.
Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J 2009; 28: 1843–1854.
DiFeo A, Feld L, Rodriguez E, Wang C, Beer DG, Martignetti JA et al. A functional role for KLF6-SV1 in lung adenocarcinoma prognosis and chemotherapy response. Cancer Res 2008; 68: 965–970.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Bienz M, Clevers H . Linking colorectal cancer to Wnt signaling. Cell 2000; 103: 311–320.
Cadigan KM . TCFs and Wnt/beta-catenin signaling: more than one way to throw the switch. Curr Top Dev Biol 2012; 98: 1–34.
Foglietti C, Filocamo G, Cundari E, De Rinaldis E, Lahm A, Cortese R et al. Dissecting the biological functions of Drosophila histone deacetylases by RNA interference and transcriptional profiling. J Biol Chem 2006; 281: 17968–17976.
Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F . C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J 2000; 19: 612–622.
Sangodkar J, Shi J, DiFeo A, Schwartz R, Bromberg R, Choudhri A et al. Functional role of the KLF6 tumour suppressor gene in gastric cancer. Eur J Cancer 2009; 45: 666–676.
Brachmann CB, Jassim OW, Wachsmuth BD, Cagan RL . The Drosophila bcl-2 family member dBorg-1 functions in the apoptotic response to UV-irradiation. Curr Biol 2000; 10: 547–550.
Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengelbach M et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA 2001; 98: 13784–13789.
Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med 2001; 194: 1639–1647.
Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002; 13: 1929–1939.
Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA et al. Molecular portraits of human breast tumours. Nature 2000; 406: 747–752.
Higgins JP, Shinghal R, Gill H, Reese JH, Terris M, Cohen RJ et al. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am J Pathol 2003; 162: 925–932.
Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell 2003; 14: 3208–3215.
Acknowledgements
We thank members of the Cagan Lab for sharing reagents, information, and helpful advice and Justin Graves for providing help mapping UAS-dRetMEN2 transgenic flies. This work was supported by NIH/NCI grants R01-CA084309 and R01-CA109730 to RC and American Cancer Society Fellowship grant 120886-PFM-11-137-01-DDC to TD.
Author contributions: T.D. and R.C. designed the project. T.D. performed the in vivo Drosophila work, the transient siRNA transfection and western analysis on cell lines, and analyzed the ONCOMINE data. T.D. and J.S. performed wound healing assay. J.S. performed MTT assay. G.N. and J.S. generated and analyzed patient tumor expression data. N.N. generated the ChIP-seq data. T.D. and R.C. analyzed the data and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Das, T., Sangodkar, J., Negre, N. et al. Sin3a acts through a multi-gene module to regulate invasion in Drosophila and human tumors. Oncogene 32, 3184–3197 (2013). https://doi.org/10.1038/onc.2012.326
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.326
Keywords
This article is cited by
-
Suppression of the long non-coding RNA LINC01279 triggers autophagy and apoptosis in lung cancer by regulating FAK and SIN3A
Discover Oncology (2024)
-
Bioinformatics analysis of the pathogenic link between Epstein-Barr virus infection, systemic lupus erythematosus and diffuse large B cell lymphoma
Scientific Reports (2023)
-
Identification of potential key genes and miRNAs involved in Hepatoblastoma pathogenesis and prognosis
Journal of Cell Communication and Signaling (2021)
-
Berberine chloride suppresses non-small cell lung cancer by deregulating Sin3A/TOP2B pathway in vitro and in vivo
Cancer Chemotherapy and Pharmacology (2020)
-
Genome-wide studies reveal novel and distinct biological pathways regulated by SIN3 isoforms
BMC Genomics (2016)