Abstract
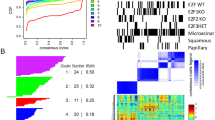
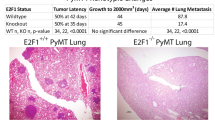
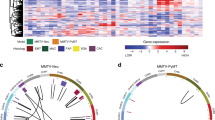
Metastasis is the primary cause of death in patients with breast cancer. Overexpression of c-myc in humans correlates with metastases, but transgenic mice only show low rates of micrometastases. We have generated transgenic mice that overexpress both c-myc and vascular endothelial growth factor (VEGF) (Myc/VEGF) in the mammary gland, which develop high rates of pulmonary macrometastases. Gene expression profiling revealed a set of deregulated genes in Myc/VEGF tumors compared to Myc tumors associated with the increased metastatic phenotype. Cross-comparisons between this set of genes with a human breast cancer lung metastasis gene signature identified five common targets: tenascin-C(TNC), matrix metalloprotease-2, collagen-6-A1, mannosidase-α-1A and HLA-DPA1. Signaling blockade or knockdown of TNC in MDA-MB-435 cells resulted in a significant impairment of cell migration and anchorage-independent cell proliferation. Mice injected with clonal MDA-MB-435 cells with reduced expression of TNC demonstrated a significant decrease (P<0.05) in (1) primary tumor growth; (2) tumor relapse after surgical removal of the primary tumor and (3) incidence of lung metastasis. Our results demonstrate that VEGF induces complex alterations in tissue architecture and gene expression. The TNC signaling pathway plays an important role in mammary tumor growth and metastases, suggesting that TNC may be a relevant target for therapy against metastatic breast cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V et al. (2000). Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res 60: 2898–2905.
Amundadottir LT, Johnson MD, Merlino G, Smith GH, Dickson RB . (1995). Synergistic interaction of transforming growth factor alpha and c-myc in mouse mammary and salivary gland tumorigenesis. Cell Growth Differ 6: 737–748.
Calvo A, Xiao N, Kang J, Best CJ, Leiva I, Emmert-Buck MR et al. (2002). Alterations in gene expression profiles during prostate cancer progression: functional correlations to tumorigenicity and down-regulation of selenoprotein-P in mouse and human tumors. Cancer Res 62: 5325–5335.
Chiquet-Ehrismann R, Chiquet M . (2003). Tenascins: regulation and putative functions during pathological stress. J Pathol 200: 488–499.
De Paola F, Granato AM, Scarpi E, Monti F, Medri L, Bianchi S et al. (2002). Vascular endothelial growth factor and prognosis in patients with node-negative breast cancer. Int J Cancer 98: 228–233.
Deming SL, Nass SJ, Dickson RB, Trock BJ . (2000). C-myc amplification in breast cancer: a meta-analysis of its occurrence and prognostic relevance. Br J Cancer 83: 1688–1695.
Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD et al. (2005). Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res 3: 1–13.
Ferrara N . (1999). Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int 56: 794–814.
Folkman J . (2002). Role of angiogenesis in tumor growth and metastasis. Semin Oncol 29 (6 Suppl 16): 15–18.
Giannelli G, Antonaci S . (2000). Biological and clinical relevance of Laminin-5 in cancer. Clin Exp Metastasis 18: 439–443.
Gupta GP, Massague J . (2006). Cancer metastasis: building a framework. Cell 127: 679–695.
Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C et al. (2007). Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature 446: 765–770.
Huh JI, Calvo A, Stafford J, Cheung M, Kumar R, Philp D et al. (2005). Inhibition of VEGF receptors significantly impairs mammary cancer growth in C3(1)/Tag transgenic mice through antiangiogenic and non-antiangiogenic mechanisms. Oncogene 24: 790–800.
Hundley JE, Koester SK, Troyer DA, Hilsenbeck SG, Barrington RE, Windle JJ . (1997). Differential regulation of cell cycle characteristics and apoptosis in MMTV-myc and MMTV-ras mouse mammary tumors. Cancer Res 57: 600–603.
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ . (2007). Cancer statistics, 2007. CA Cancer J Clin 57: 43–66.
Jhappan C, Stahle C, Harkins RN, Fausto N, Smith GH, Merlino GT . (1990). TGF alpha overexpression in transgenic mice induces liver neoplasia and abnormal development of the mammary gland and pancreas. Cell 61: 1137–1146.
Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T et al. (2003). Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor beta1. Int J Cancer 105: 53–60.
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature 436: 518–524.
Miralem T, Steinberg R, Price D, Avraham H . (2001). VEGF(165) requires extracellular matrix components to induce mitogenic effects and migratory response in breast cancer cells. Oncogene 20: 5511–5524.
Miyazaki K . (2006). Unique biological activity and role in tumor growth and invasion. Cancer Sci 97: 91–98.
Noel A, Maillard C, Rocks N, Jost M, Chabottaux V, Sounni NE et al. (2004). Membrane associated proteases and their inhibitors in tumour angiogenesis. J Clin Pathol 57: 577–584.
Pei XF, Noble MS, Davoli MA, Rosfjord E, Tilli MT, Furth PA et al. (2004). Explant-cell culture of primary mammary tumors from MMTV-c-Myc transgenic mice. In vitro Cell Dev Biol Anim 40: 14–21.
Pyke C, Romer J, Kallunki P, Lund LR, Ralfkiaer E, Dano K et al. (1994). The gamma 2 chain of kalinin/laminin 5 is preferentially expressed in invading malignant cells in human cancers. Am J Pathol 145: 782–791.
Qiu TH, Chandramouli GV, Hunter KW, Alkharouf NW, Green JE, Liu ET . (2004). Global expression profiling identifies signatures of tumor virulence in MMTV-PyMT-transgenic mice: correlation to human disease. Cancer Res 64: 5973–5981.
Ramaswamy S, Ross KN, Lander ES, Golub TR . (2003). A molecular signature of metastasis in primary solid tumors. Nat Genet 33: 49–54.
Schmitz AA, Govek EE, Bottner B, Van Aelst L . (2000). Rho GTPases: signaling, migration, and invasion. Exp Cell Res 261: 1–12.
Schoeffner DJ, Matheny SL, Akahane T, Factor V, Berry A, Merlino G et al. (2005). VEGF contributes to mammary tumor growth in transgenic mice through paracrine and autocrine mechanisms. Lab Invest 85: 608–623.
Shih SC, Robinson GS, Perruzzi CA, Calvo A, Desai K, Green JE et al. (2002). Molecular profiling of angiogenesis markers. Am J Pathol 161: 35–41.
Stamenkovic I . (2000). Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol 10: 415–433.
Suyama E, Kawasaki H, Nakajima M, Taira K . (2003). Identification of genes involved in cell invasion by using a library of randomized hybrid ribozymes. Proc Natl Acad Sci USA 100: 5616–5621. Epub 28 April 2003.
Talts JF, Wirl G, Dictor M, Muller WJ, Fassler R . (1999). Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J Cell Sci 112 (Pt 12): 1855–1864.
Tsunoda T, Inada H, Kalembeyi I, Imanaka-Yoshida K, Sakakibara M, Okada R et al. (2003). Involvement of large tenascin-C splice variants in breast cancer progression. Am J Pathol 162: 1857–1867.
Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K . (2001). Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin Cancer Res 7: 896–900.
Zagzag D, Capo V . (2002). Angiogenesis in the central nervous system: a role for vascular endothelial growth factor/vascular permeability factor and tenascin-C. Common molecular effectors in cerebral neoplastic and non-neoplastic ‘angiogenic diseases’. Histol Histopathol 17: 301–321.
Zagzag D, Shiff B, Jallo GI, Greco MA, Blanco C, Cohen H et al. (2002). Tenascin-C promotes microvascular cell migration and phosphorylation of focal adhesion kinase. Cancer Res 62: 2660–2668.
Acknowledgements
We would like to thank Ms Mercedes Calvo for manuscript preparation, Victor Segura and Elizabeth Guruceaga (CEIT and Tecnun, Pamplona, Spain) for their help in bioinformatic analysis, GlaxoSmithKline for the GW654652 compound, Dr René Bernards (The Netherlands Cancer Institute) for the pMSCV-△31LTR vector, Dr Linda Metheny-Barlow (at the LCCC) for helpful discussions, Edward C Rosfjord and Sandra L Deming and Gloria Chepko for contributions to the initial Myc/VEGF crossbreeding study. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, with funds from NIH (to GM) under Contract No. NO1-CO-12400, a grant from the Susan G Komen Foundation (to RBD), a grant from the DOD Breast Cancer Program DAMD17-01-1-0255 (to MDJ), NIH R01 CA72460 (to RBD), NIH 2R01 CA104963 (to RBD) ISCIII-RETIC (RD06/0020, to AC) and a Fulbright-MEC fellowship (AC); O G-M was supported by a Spanish MEC fellowship, and RBD by an NIH grant 2 R01 AG14963-06.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Rights and permissions
About this article
Cite this article
Calvo, A., Catena, R., Noble, M. et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene 27, 5373–5384 (2008). https://doi.org/10.1038/onc.2008.155
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2008.155
Keywords
This article is cited by
-
The genomic architecture of metastasis in breast cancer: focus on mechanistic aspects, signalling pathways and therapeutic strategies
Medical Oncology (2021)
-
Assessment of the WAP-Myc mouse mammary tumor model for spontaneous metastasis
Scientific Reports (2020)
-
Periostin and tenascin-C interaction promotes angiogenesis in ischemic proliferative retinopathy
Scientific Reports (2020)
-
Tenascin-C expression contributes to pediatric brainstem glioma tumor phenotype and represents a novel biomarker of disease
Acta Neuropathologica Communications (2019)
-
Targeted matrisome analysis identifies thrombospondin-2 and tenascin-C in aligned collagen stroma from invasive breast carcinoma
Scientific Reports (2018)