Abstract
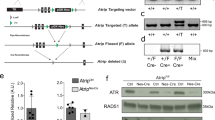
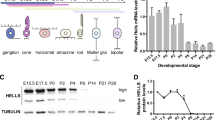
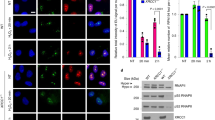
Lens epithelium–derived growth factor p75 splice variant (LEDGF) is a chromatin-binding protein known for its antiapoptotic activity and ability to direct human immunodeficiency virus into active transcription units. Here we show that LEDGF promotes the repair of DNA double-strand breaks (DSBs) by the homologous recombination repair pathway. Depletion of LEDGF impairs the recruitment of C-terminal binding protein interacting protein (CtIP) to DNA DSBs and the subsequent CtIP-dependent DNA-end resection. LEDGF is constitutively associated with chromatin through its Pro-Trp-Trp-Pro (PWWP) domain that binds preferentially to epigenetic methyl-lysine histone markers characteristic of active transcription units. LEDGF binds CtIP in a DNA damage–dependent manner, thereby enhancing its tethering to the active chromatin and facilitating its access to DNA DSBs. These data highlight the role of PWWP-domain proteins in DNA repair and provide a molecular explanation for the antiapoptotic and cancer cell survival–activities of LEDGF.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Daugaard, M. et al. Lens epithelium-derived growth factor is an Hsp70–2 regulated guardian of lysosomal stability in human cancer. Cancer Res. 67, 2559–2567 (2007).
Llano, M. et al. Identification and characterization of the chromatin-binding domains of the HIV-1 integrase interactor LEDGF/p75. J. Mol. Biol. 360, 760–773 (2006).
Ge, H., Si, Y. & Wolffe, A.P. A novel transcriptional coactivator, p52, functionally interacts with the essential splicing factor ASF/SF2. Mol. Cell 2, 751–759 (1998).
Brown-Bryan, T.A. et al. Alternative splicing and caspase-mediated cleavage generate antagonistic variants of the stress oncoprotein LEDGF/p75. Mol. Cancer Res. 6, 1293–1307 (2008).
Nishizawa, Y., Usukura, J., Singh, D.P., Chylack, L.T. Jr. & Shinohara, T. Spatial and temporal dynamics of two alternatively spliced regulatory factors, lens epithelium-derived growth factor (ledgf/p75) and p52, in the nucleus. Cell Tissue Res. 305, 107–114 (2001).
Dietz, F. et al. The family of hepatoma-derived growth factor proteins: characterization of a new member HRP-4 and classification of its subfamilies. Biochem. J. 366, 491–500 (2002).
Sutherland, H.G. et al. Disruption of ledgf/psip1 results in perinatal mortality and homeotic skeletal transformations. Mol. Cell. Biol. 26, 7201–7210 (2006).
Bartholomeeusen, K. et al. Lens epithelium derived growth factor/p75 interacts with the transposase derived DDE domain of pogZ. J. Biol. Chem. 284, 11467–11477 (2009).
Hughes, S., Jenkins, V., Dar, M.J., Engelman, A. & Cherepanov, P. Transcriptional co-activator LEDGF interacts with Cdc7-activator of S-phase kinase (ASK) and stimulates its enzymatic activity. J. Biol. Chem. 285, 541–554 (2010).
Maertens, G.N., Cherepanov, P. & Engelman, A. Transcriptional co-activator p75 binds and tethers the Myc-interacting protein JPO2 to chromatin. J. Cell Sci. 119, 2563–2571 (2006).
Yokoyama, A. & Cleary, M.L. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell 14, 36–46 (2008).
Ciuffi, A. et al. A role for LEDGF/p75 in targeting HIV DNA integration. Nat. Med. 11, 1287–1289 (2005).
Shun, M.C. et al. Identification and characterization of PWWP domain residues critical for LEDGF/p75 chromatin binding and human immunodeficiency virus type 1 infectivity. J. Virol. 82, 11555–11567 (2008).
Ciuffi, A. Mechanisms governing lentivirus integration site selection. Curr. Gene Ther. 8, 419–429 (2008).
De Rijck, J., Bartholomeeusen, K., Ceulemans, H., Debyser, Z. & Gijsbers, R. High-resolution profiling of the LEDGF/p75 chromatin interaction in the ENCODE region. Nucleic Acids Res. 38, 6135–6147 (2010).
De Luca, L. et al. Small molecules targeting the interaction between HIV-1 integrase and LEDGF/p75 cofactor. Bioorg. Med. Chem. 18, 7515–7521 (2010).
Vets, S. et al. Lens epithelium-derived growth factor/p75 qualifies as a target for HIV gene therapy in the NSG mouse model. Mol. Ther. 20, 908–917 (2012).
De Luca, L., Ferro, S., Morreale, F. & Chimirri, A. Inhibition of the interaction between HIV-1 integrase and its cofactor LEDGF/p75: a promising approach in anti-retroviral therapy. Mini Rev. Med. Chem. 11, 714–727 (2011).
Hussey, D.J., Moore, S., Nicola, M. & Dobrovic, A. Fusion of the NUP98 gene with the LEDGF/p52 gene defines a recurrent acute myeloid leukemia translocation. BMC Genet. 2, 20 (2001).
Daniels, T. et al. Antinuclear autoantibodies in prostate cancer: immunity to LEDGF/p75, a survival protein highly expressed in prostate tumors and cleaved during apoptosis. Prostate 62, 14–26 (2005).
Matsui, H., Lin, L.R., Singh, D.P., Shinohara, T. & Reddy, V.N. Lens epithelium-derived growth factor: increased survival and decreased DNA breakage of human RPE cells induced by oxidative stress. Invest. Ophthalmol. Vis. Sci. 42, 2935–2941 (2001).
Wang, Y. et al. Regulation of Set9-mediated H4K20 methylation by a PWWP domain protein. Mol. Cell 33, 428–437 (2009).
Laguri, C. et al. Human mismatch repair protein MSH6 contains a PWWP domain that targets double stranded DNA. Biochemistry 47, 6199–6207 (2008).
Huen, M.S. et al. Regulation of chromatin architecture by the PWWP domain-containing DNA damage-responsive factor EXPAND1/MUM1. Mol. Cell 37, 854–864 (2010).
Kousholt, A.N. et al. CtIP-dependent DNA resection is required for DNA damage checkpoint maintenance but not initiation. J. Cell Biol. (in the press).
Moynahan, M.E., Cui, T.Y. & Jasin, M. Homology-directed DNA repair, mitomycin-c resistance, and chromosome stability is restored with correction of a Brca1 mutation. Cancer Res. 61, 4842–4850 (2001).
Sartori, A.A. et al. Human CtIP promotes DNA end resection. Nature 450, 509–514 (2007).
Spencer, D.M. et al. DNA repair in response to anthracycline-DNA adducts: a role for both homologous recombination and nucleotide excision repair. Mutat. Res. 638, 110–121 (2008).
Caldecott, K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 9, 619–631 (2008).
Jackson, S.P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461, 1071–1078 (2009).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bryant, H.E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Pierce, A.J., Hu, P., Han, M., Ellis, N. & Jasin, M. Ku DNA end-binding protein modulates homologous repair of double-strand breaks in mammalian cells. Genes Dev. 15, 3237–3242 (2001).
Huertas, P. DNA resection in eukaryotes: deciding how to fix the break. Nat. Struct. Mol. Biol. 17, 11–16 (2010).
Garcia, V., Phelps, S.E.L., Gray, S. & Neale, M.J. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature 479, 241–244 (2011).
Bernstein, K.A. & Rothstein, R. At loose ends: resecting a double-strand break. Cell 137, 807–810 (2009).
Bekker-Jensen, S. et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J. Cell Biol. 173, 195–206 (2006).
Shao, R.G. et al. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 18, 1397–1406 (1999).
Manthey, K.C. et al. NBS1 mediates ATR-dependent RPA hyperphosphorylation following replication-fork stall and collapse. J. Cell Sci. 120, 4221–4229 (2007).
You, Z. et al. CtIP links DNA double-strand break sensing to resection. Mol. Cell 36, 954–969 (2009).
Lukas, C., Falck, J., Bartkova, J., Bartek, J. & Lukas, J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5, 255–260 (2003).
Cherepanov, P. et al. Solution structure of the HIV-1 integrase-binding domain in LEDGF/p75. Nat. Struct. Mol. Biol. 12, 526–532 (2005).
Vezzoli, A. et al. Molecular basis of histone H3K36me3 recognition by the PWWP domain of Brpf1. Nat. Struct. Mol. Biol. 17, 617–619 (2010).
Dhayalan, A. et al. The DNMT3A PWWP domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem. 285, 26114–26120 (2010).
Saunders, A., Core, L.J. & Lis, J.T. Breaking barriers to transcription elongation. Nat. Rev. Mol. Cell Biol. 7, 557–567 (2006).
Liu, F. & Lee, W.H. CtIP activates its own and cyclin D1 promoters via the E2F/RB pathway during G1/S progression. Mol. Cell. Biol. 26, 3124–3134 (2006).
Bennardo, N., Cheng, A., Huang, N. & Stark, J.M. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 4, e1000110 (2008).
Chen, P.L. et al. Inactivation of CtIP leads to early embryonic lethality mediated by G1 restraint and to tumorigenesis by haploid insufficiency. Mol. Cell. Biol. 25, 3535–3542 (2005).
Lim, D.S. & Hasty, P. A mutation in mouse rad51 results in an early embryonic lethal that is suppressed by a mutation in p53. Mol. Cell. Biol. 16, 7133–7143 (1996).
Wang, Y. et al. Mutation in Rpa1 results in defective DNA double-strand break repair, chromosomal instability and cancer in mice. Nat. Genet. 37, 750–755 (2005).
Vermeulen, M. et al. Quantitative interaction proteomics and genome-wide profiling of epigenetic histone marks and their readers. Cell 142, 967–980 (2010).
Skalka, A.M. & Katz, R.A. Retroviral DNA integration and the DNA damage response. Cell Death Differ. 12 (suppl. 1), 971–978 (2005).
Katz, R.A., Greger, J.G. & Skalka, A.M. Effects of cell cycle status on early events in retroviral replication. J. Cell. Biochem. 94, 880–889 (2005).
Sinclair, A., Yarranton, S. & Schelcher, C. DNA-damage response pathways triggered by viral replication. Expert Rev. Mol. Med. 8, 1–11 (2006).
Pommier, Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6, 789–802 (2006).
Llorente, B., Smith, C.E. & Symington, L.S. Break-induced replication: what is it and what is it for? Cell Cycle 7, 859–864 (2008).
Lukas, C., Falck, J., Bartkova, J., Bartek, J. & Lukas, J. Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage. Nat. Cell Biol. 5, 255–260 (2003).
Beck, H. et al. Regulators of cyclin-dependent kinases are crucial for maintaining genome integrity in S phase. J. Cell Biol. 188, 629–638 (2010).
Huang, W., Sherman, B.T. & Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Acknowledgements
We thank A. Engelman (Dana-Farber Cancer Institute, Harvard Medical School) for providing us with Psip1-deficient cells, K.G. Henriksen for technical assistance and L. Vinner for discussions regarding HIV integration. This work was supported by the Danish Cancer Society and the Novo Nordisk Foundation (M.D., J.L., J.B. and M.J.), the Danish National Research Foundation and the John and Birthe Meyer Foundation (J.L., J.B. and M.J.), the Danish Medical Research Council, the Lundbeck Foundation and the European Commission FP7 (APO-SYS) (M.J.), and DDResponse, Biomedreg and INFLA-CARE (J.B).
Author information
Authors and Affiliations
Contributions
M.D. and M.J. designed the project and wrote the manuscript; M.D. and A.B. performed most of the experiments, supported by K.F., L.K.P., H.B., N.H.T.P. and M.R.; and C.S.S., C.L., J.L., J.B. and P.H.B.S. provided essential knowhow and support for the project and for preparing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 2783 kb)
Rights and permissions
About this article
Cite this article
Daugaard, M., Baude, A., Fugger, K. et al. LEDGF (p75) promotes DNA-end resection and homologous recombination. Nat Struct Mol Biol 19, 803–810 (2012). https://doi.org/10.1038/nsmb.2314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2314
This article is cited by
-
PSIP1/LEDGF reduces R-loops at transcription sites to maintain genome integrity
Nature Communications (2024)
-
Structural and functional specificity of H3K36 methylation
Epigenetics & Chromatin (2022)
-
H3K36 trimethylation-mediated biological functions in cancer
Clinical Epigenetics (2021)
-
H3K36me3, message from chromatin to DNA damage repair
Cell & Bioscience (2020)
-
Histone methyltransferase and drug resistance in cancers
Journal of Experimental & Clinical Cancer Research (2020)