Key Points
-
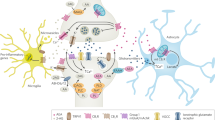
Endocannabinoid (eCB) signalling contributes to activity-dependent modulation of synaptic activity in all brain regions involved in emotional regulation. As such, it is an integral part of the interface between stimulus input and responses at the synaptic level, thereby regulating behavioural responses.
-
eCB signalling exerts a buffering effect on neuronal activity in specific brain circuits within specific limits. Thus, a minimal stimulus is required to activate eCB signalling, and intense stimulation can overwhelm the buffering capacity.
-
The predominant effect of cannabinoid receptor type 1 (CB1R) activation is to reduce anxiety; however, CB1R agonists have bidirectional effects on anxiety-like behaviours. Recent data indicate that CB1R signalling modulates both pro- and anti-anxiety pathways and thus contributes broadly to regulation of anxiety-like behaviours.
-
eCB signalling in the amygdala and other brain regions is essential for several aspects of fear-memory processing, most prominently for extinction of fear responses. Available data suggest that repeated re-exposure to a fear-related stimulus in the absence of the threat increasingly activates eCB signalling, finally contributing to habituation and/or extinction of specific fear responses.
-
The eCB system seems to exert bidirectional control of the choice of coping strategy in response to a threatening stimulus, depending on the neuronal types and circuits involved.
-
eCB signalling is altered by stress and it is centrally involved in the effects of stress and glucocorticoids on synaptic activity.
-
The effect of the eCB system on the processing of anxiety, fear and stress makes it a tantalizing target for the therapeutic treatment of several psychopathologies that occur as a result of inappropriate emotional regulation. However, realizing this potential will not be an easy task, given the complexity of the eCB system.
Abstract
The endocannabinoid (eCB) system has emerged as a central integrator linking the perception of external and internal stimuli to distinct neurophysiological and behavioural outcomes (such as fear reaction, anxiety and stress-coping), thus allowing an organism to adapt to its changing environment. eCB signalling seems to determine the value of fear-evoking stimuli and to tune appropriate behavioural responses, which are essential for the organism's long-term viability, homeostasis and stress resilience; and dysregulation of eCB signalling can lead to psychiatric disorders. An understanding of the underlying neural cell populations and cellular processes enables the development of therapeutic strategies to mitigate behavioural maladaptation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tart, C. T. Marijuana intoxication common experiences. Nature 226, 701–704 (1970).
Lafenetre, P., Chaouloff, F. & Marsicano, G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol. Res. 56, 367–381 (2007).
Riebe, C. J., Pamplona, F. A., Kamprath, K. & Wotjak, C. T. Fear relief—toward a new conceptual frame work and what endocannabinoids gotta do with it. Neuroscience 204, 159–185 (2012).
Ruehle, S., Rey, A. A., Remmers, F. & Lutz, B. The endocannabinoid system in anxiety, fear memory and habituation. J. Psychopharmacol. 26, 23–39 (2012).
Akirav, I. The role of cannabinoids in modulating emotional and non-emotional memory processes in the hippocampus. Front. Behav. Neurosci. 5, 34 (2011).
Gunduz-Cinar, O., Hill, M. N., McEwen, B. S. & Holmes, A. Amygdala FAAH and anandamide: mediating protection and recovery from stress. Trends Pharmacol. Sci. 34, 637–644 (2013).
McLaughlin, R. J., Hill, M. N. & Gorzalka, B. B. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 42, 116–131 (2014).
Castillo, P. E., Younts, T. J., Chavez, A. E. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012).
Katona, I. & Freund, T. F. Multiple functions of endocannabinoid signaling in the brain. Annu. Rev. Neurosci. 35, 529–558 (2012).
Marsicano, G. & Kuner, R. in Anatomical Distribution of Receptors, Ligands and Enzymes in the Brain and in the Spinal Cord: Circuitries and Neurochemistry in Cannabinoids in the Brain (ed. Köfalvi, A.) 161–201 (Springer, 2008).
Metna-Laurent, M. & Marsicano, G. Rising stars: modulation of brain functions by astroglial type-1 cannabinoid receptors. Glia 63, 353–364 (2015).
Stella, N. Endocannabinoid signaling in microglial cells. Neuropharmacology 56, 244–253 (2009).
Alger, B. E. & Kim, J. Supply and demand for endocannabinoids. Trends Neurosci. 34, 304–315 (2011).
Ohno-Shosaku, T. & Kano, M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr. Opin. Neurobiol. 29, 1–8 (2014).
Soltesz, I. et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat. Rev. Neurosci. 16, 264–277 (2015).
Marsicano, G. & Lutz, B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci. 11, 4213–4225 (1999).
Katona, I. et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J. Neurosci. 19, 4544–4558 (1999).
Lafourcade, M. et al. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS ONE 2, e709 (2007).
Puente, N. et al. Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS ONE 5, e8869 (2010).
Katona, I. et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J. Neurosci. 21, 9506–9518 (2001).
Kamprath, K. et al. Short-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdala. Neuropsychopharmacology 36, 652–663 (2011).
Ramikie, T. S. et al. Multiple mechanistically distinct modes of endocannabinoid mobilization at central amygdala glutamatergic synapses. Neuron 81, 1111–1125 (2014).
Cristino, L. et al. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 139, 1405–1415 (2006).
Marsicano, G. et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88 (2003).
Marinelli, S., Pacioni, S., Cannich, A., Marsicano, G. & Bacci, A. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat. Neurosci. 12, 1488–1490 (2009).
Kirilly, E., Hunyady, L. & Bagdy, G. Opposing local effects of endocannabinoids on the activity of noradrenergic neurons and release of noradrenaline: relevance for their role in depression and in the actions of CB1 receptor antagonists. J. Neural Transm. 120, 177–186 (2013).
Häring, M., Marsicano, G., Lutz, B. & Monory, K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience 146, 1212–1219 (2007).
Häring, M. et al. Cannabinoid type-1 receptor signaling in central serotonergic neurons regulates anxiety-like behavior and sociability. Front. Behav. Neurosci. 9, 235 (2015).
Nyiri, G. et al. GABAB and CB1 cannabinoid receptor expression identifies two types of septal cholinergic neurons. Eur. J. Neurosci. 21, 3034–3042 (2005).
Han, J. et al. Acute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTD. Cell 148, 1039–1050 (2012).
Carta, M. et al. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron 81, 787–799 (2014).
Piomelli, D. et al. Dopamine activation of the arachidonic acid cascade as a basis for D1/D2 receptor synergism. Nature 353, 164–167 (1991).
Meves, H. Arachidonic acid and ion channels: an update. Br. J. Pharmacol. 155, 4–16 (2008).
Shonesy, B. C. et al. Genetic disruption of 2-arachidonoylglycerol synthesis reveals a key role for endocannabinoid signaling in anxiety modulation. Cell Rep. 9, 1644–1653 (2014).
Schlosburg, J. E. et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 13, 1113–1119 (2010).
Jenniches, I. et al. Anxiety, stress and fear response in mice with reduced endocannabinoid levels. Biol. Psych http://dx.doi.org/10.1016/j.biopsych.2015.03.033 (2015).
Navarrete, M., Diez, A. & Araque, A. Astrocytes in endocannabinoid signalling. Phil. Trans. R. Soc. B 369, 20130599 (2014).
Lutz, B. Endocannabinoid signals in the control of emotion. Curr. Opin. Pharmacol. 9, 46–52 (2009).
Di, S. et al. Activity-dependent release and actions of endocannabinoids in the rat hypothalamic supraoptic nucleus. J. Physiol. 569, 751–760 (2005).
Hill, M. N., Karatsoreos, I. N., Hillard, C. J. & McEwen, B. S. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 35, 1333–1338 (2010).
Huang, G. Z. & Woolley, C. S. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron 74, 801–808 (2012).
Tovote, P., Fadok, J. P. & Luthi, A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331 (2015).
Bains, J. S., Cusulin, J. I. & Inoue, W. Stress-related synaptic plasticity in the hypothalamus. Nat. Rev. Neurosci. 16, 377–388 (2015).
Graham, B. M., Langton, J. M. & Richardson, R. Pharmacological enhancement of fear reduction: preclinical models. Br. J. Pharmacol. 164, 1230–1247 (2011).
Sylvers, P., Lilienfeld, S. O. & LaPrairie, J. L. Differences between trait fear and trait anxiety: implications for psychopathology. Clin. Psychol. Rev. 31, 122–137 (2011).
Mechoulam, R. & Parker, L. A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 64, 21–47 (2013).
Jacob, W. et al. Endocannabinoids render exploratory behaviour largely independent of the test aversiveness: role of glutamatergic transmission. Genes Brain Behav. 8, 685–698 (2009).
Sumislawski, J. J., Ramikie, T. S. & Patel, S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 36, 2750–2761 (2011).
Rey, A. A., Purrio, M., Viveros, M. P. & Lutz, B. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABAB receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37, 2624–2634 (2012).
Häring, M., Kaiser, N., Monory, K. & Lutz, B. Circuit specific functions of cannabinoid CB1 receptor in the balance of investigatory drive and exploration. PLoS ONE 6, e26617 (2011).
Lafenetre, P., Chaouloff, F. & Marsicano, G. Bidirectional regulation of novelty-induced behavioral inhibition by the endocannabinoid system. Neuropharmacology 57, 715–721 (2009).
Legler, A., Monory, K. & Lutz, B. Age differences in the role of the cannabinoid type 1 receptor on glutamatergic neurons in habituation and spatial memory acquisition. Life Sci. 138, 63 (2015).
Ruehle, S. et al. Cannabinoid CB1 receptor in dorsal telencephalic glutamatergic neurons: distinctive sufficiency for hippocampus-dependent and amygdala-dependent synaptic and behavioral functions. J. Neurosci. 33, 10264–10277 (2013).
Moreira, F. A., Grieb, M. & Lutz, B. Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best. Pract. Res. Clin. Endocrinol. Metab. 23, 133–144 (2009).
Rubino, T. et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology 54, 151–160 (2008).
Monory, K., Polack, M., Remus, A., Lutz, B. & Korte, M. Cannabinoid CB1 receptor calibrates excitatory synaptic balance in the mouse hippocampus. J. Neurosci. 35, 3842–3850 (2015).
Moreira, F. A., Kaiser, N., Monory, K. & Lutz, B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology 54, 141–150 (2008).
Kathuria, S. et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 9, 76–81 (2003). This landmark publication shows the high efficacy of drugs that inhibit AEA hydrolysis and their potential use in the treatment of anxiety-related disorder.
Patel, S. & Hillard, C. J. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J. Pharmacol. Exp. Ther. 318, 304–311 (2006).
Lomazzo, E. et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 40, 488–501 (2015).
Sipe, J. C., Chiang, K., Gerber, A. L., Beutler, E. & Cravatt, B. F. A missense mutation in human fatty acid amide hydrolase associated with problem drug use. Proc. Natl Acad. Sci. USA 99, 8394–8399 (2002).
Dincheva, I. et al. FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat. Commun. 6, 6395 (2015).
Guggenhuber, S. et al. Impaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibility. Int. J. Neuropsychopharmacol. http://dx.doi.org/10.1093/ijnp/pyv091 (2015).
Busquets-Garcia, A. et al. Differential role of anandamide and 2-arachidonoylglycerol in memory and anxiety-like responses. Biol. Psychiatry 70, 479–486 (2011). This study shows that anxiolytic-like effects of AEA are mediated by CB1R and associated with memory disruption, whereas 2-AG induces an anxiolytic effect via CB2R without affecting cognitive functions.
Kinsey, S. G., O'Neal, S. T., Long, J. Z., Cravatt, B. F. & Lichtman, A. H. Inhibition of endocannabinoid catabolic enzymes elicits anxiolytic-like effects in the marble burying assay. Pharmacol. Biochem. Behav. 98, 21–27 (2011).
Sciolino, N. R., Zhou, W. & Hohmann, A. G. Enhancement of endocannabinoid signaling with JZL184, an inhibitor of the 2-arachidonoylglycerol hydrolyzing enzyme monoacylglycerol lipase, produces anxiolytic effects under conditions of high environmental aversiveness in rats. Pharmacol. Res. 64, 226–234 (2011).
Zhong, P. et al. Monoacylglycerol lipase inhibition blocks chronic stress-induced depressive-like behaviors via activation of mTOR signaling. Neuropsychopharmacology 39, 1763–1776 (2014).
Marsch, R. et al. Reduced anxiety, conditioned fear, and hippocampal long-term potentiation in transient receptor potential vanilloid type 1 receptor-deficient mice. J. Neurosci. 27, 832–839 (2007).
Sun, F. J. et al. Increased expression of TRPV1 in the cortex and hippocampus from patients with mesial temporal lobe epilepsy. J. Mol. Neurosci. 49, 182–193 (2013).
Puente, N. et al. The transient receptor potential vanilloid-1 is localized at excitatory synapses in the mouse dentate gyrus. Brain Struct. Funct. 220, 1187–1194 (2015).
Chavez, A. E., Hernandez, V. M., Rodenas-Ruano, A., Chan, C. S. & Castillo, P. E. Compartment-specific modulation of GABAergic synaptic transmission by TRPV1 channels in the dentate gyrus. J. Neurosci. 34, 16621–16629 (2014).
Chavez, A. E., Chiu, C. Q. & Castillo, P. E. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat. Neurosci. 13, 1511–1518 (2010).
Bahi, A. et al. β-caryophyllene, a CB2 receptor agonist produces multiple behavioral changes relevant to anxiety and depression in mice. Physiol. Behav. 135, 119–124 (2014).
Busquets-Garcia, A. et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat. Med. 19, 603–607 (2013).
Aguiar, D. C., Terzian, A. L., Guimaraes, F. S. & Moreira, F. A. Anxiolytic-like effects induced by blockade of transient receptor potential vanilloid type 1 (TRPV1) channels in the medial prefrontal cortex of rats. Psychopharmacology (Berl.) 205, 217–225 (2009).
Almeida-Santos, A. F. et al. Modulation of anxiety-like behavior by the endocannabinoid 2-arachidonoylglycerol (2-AG) in the dorsolateral periaqueductal gray. Behav. Brain Res. 252, 10–17 (2013).
Terzian, A. L., Aguiar, D. C., Guimaraes, F. S. & Moreira, F. A. Modulation of anxiety-like behaviour by transient receptor potential vanilloid type 1 (TRPV1) channels located in the dorsolateral periaqueductal gray. Eur. Neuropsychopharmacol. 19, 188–195 (2009).
Moreira, F. A., Aguiar, D. C., Terzian, A. L., Guimaraes, F. S. & Wotjak, C. T. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 204, 186–192 (2012).
LeDoux, J. E. Emotion circuits in the brain. Annu. Rev. Neurosci. 23, 155–184 (2000).
Pape, H. C. & Pare, D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol. Rev. 90, 419–463 (2010).
Nader, K. & Hardt, O. A single standard for memory: the case for reconsolidation. Nat. Rev. Neurosci. 10, 224–234 (2009).
Myers, K. M. & Davis, M. Mechanisms of fear extinction. Mol. Psychiatry 12, 120–150 (2007).
Maren, S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron 70, 830–845 (2011).
Marsicano, G. et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature 418, 530–534 (2002). This was the first study to demonstrate the effect of the eCB system on acquired fear response.
Plendl, W. & Wotjak, C. T. Dissociation of within- and between-session extinction of conditioned fear. J. Neurosci. 30, 4990–4998 (2010).
Dubreucq, S. et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology 37, 1885–1900 (2012).
Llorente-Berzal, A. et al. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology (Berl.) 232, 2811–2825 (2015).
Trouche, S., Sasaki, J. M., Tu, T. & Reijmers, L. G. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron 80, 1054–1065 (2013).
Bowers, M. E. & Ressler, K. J. Interaction between the cholecystokinin and endogenous cannabinoid systems in cued fear expression and extinction retention. Neuropsychopharmacology 40, 688–700 (2015).
Soria-Gomez, E. et al. Habenular CB receptors control the expression of aversive memories. Neuron 88, 306–313 (2015).
Pan, B. et al. Blockade of 2-arachidonoylglycerol hydrolysis by selective monoacylglycerol lipase inhibitor 4-nitrophenyl 4-(dibenzo[d][1,3]dioxol-5-yl(hydroxy)methyl)piperidine-1-carboxylate (JZL184) enhances retrograde endocannabinoid signaling. J. Pharmacol. Exp. Ther. 331, 591–597 (2009).
Pan, B. et al. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J. Neurosci. 31, 13420–13430 (2011).
Lin, H. C., Mao, S. C., Su, C. L. & Gean, P. W. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb. Cortex 19, 165–175 (2009).
Gunduz-Cinar, O. et al. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol. Psychiatry 18, 813–823 (2013). This study provides evidence for the conserved role of AEA in rodents and humans regarding amygdala function and threat processing.
Kandel, E. R. The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038 (2001).
Kamprath, K. et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J. Neurosci. 26, 6677–6686 (2006).
Kamprath, K. et al. Endocannabinoids mediate acute fear adaptation via glutamatergic neurons independently of corticotropin-releasing hormone signaling. Genes Brain Behav. 8, 203–211 (2009).
Patel, S., Roelke, C. T., Rademacher, D. J. & Hillard, C. J. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur. J. Neurosci. 21, 1057–1069 (2005).
Ganon-Elazar, E. & Akirav, I. Cannabinoid receptor activation in the basolateral amygdala blocks the effects of stress on the conditioning and extinction of inhibitory avoidance. J. Neurosci. 29, 11078–11088 (2009).
Ganon-Elazar, E. & Akirav, I. Cannabinoids and traumatic stress modulation of contextual fear extinction and GR expression in the amygdala-hippocampal-prefrontal circuit. Psychoneuroendocrinology 38, 1675–1687 (2013).
Laricchiuta, D., Centonze, D. & Petrosini, L. Effects of endocannabinoid and endovanilloid systems on aversive memory extinction. Behav. Brain Res. 256, 101–107 (2013).
Gozzi, A. et al. A neural switch for active and passive fear. Neuron 67, 656–666 (2010).
Koolhaas, J. M., de Boer, S. F., Coppens, C. M. & Buwalda, B. Neuroendocrinology of coping styles: towards understanding the biology of individual variation. Front. Neuroendocrinol. 31, 307–321 (2010).
Metna-Laurent, M. et al. Bimodal control of fear-coping strategies by CB1 cannabinoid receptors. J. Neurosci. 32, 7109–7118 (2012).
Hill, M. N. et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic–pituitary–adrenal axis. Neuropsychopharmacology 34, 2733–2745 (2009).
Gray, J. M. et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J. Neurosci. 35, 3879–3892 (2015).
Di, S., Malcher-Lopes, R., Marcheselli, V. L., Bazan, N. G. & Tasker, J. G. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146, 4292–4301 (2005). This study shows how hypothalamic eCBs and CB1R are involved in the negative feedback mechanism of the HPA axis.
Hill, M. N. et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J. Neurosci. 31, 10506–10515 (2011).
Evanson, N. K., Tasker, J. G., Hill, M. N., Hillard, C. J. & Herman, J. P. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 151, 4811–4819 (2010).
Glangetas, C. et al. Stress switches cannabinoid type-1 (CB1) receptor-dependent plasticity from LTD to LTP in the bed nucleus of the stria terminalis. J. Neurosci. 33, 19657–19663 (2013).
Crosby, K. M., Inoue, W., Pittman, Q. J. & Bains, J. S. Endocannabinoids gate state-dependent plasticity of synaptic inhibition in feeding circuits. Neuron 71, 529–541 (2011).
Hill, M. N. et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacology 30, 508–515 (2005). This study provides strong evidence that the eCB system is dysregulated under chronic stress.
Rossi, S. et al. Chronic psychoemotional stress impairs cannabinoid-receptor-mediated control of GABA transmission in the striatum. J. Neurosci. 28, 7284–7292 (2008).
Wang, W. et al. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology 35, 2249–2261 (2010).
Hillard, C. in Cannabinoids, Endocannabinoids and Stress in Cannabinoids (ed. Di Marzo, V.) 139–174 (Wiley-Blackwell, 2014).
Haj-Dahmane, S. & Shen, R. Y. Chronic stress impairs alpha1-adrenoceptor-induced endocannabinoid-dependent synaptic plasticity in the dorsal raphe nucleus. J. Neurosci. 34, 14560–14570 (2014).
Hill, M. N. et al. Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol. Psychiatry 18, 1125–1135 (2013).
Wamsteeker, J. I., Kuzmiski, J. B. & Bains, J. S. Repeated stress impairs endocannabinoid signaling in the paraventricular nucleus of the hypothalamus. J. Neurosci. 30, 11188–11196 (2010). This paper demonstrates the mechanistic underpinnings for how repeated stress interferes with eCB system activity.
Häring, M., Grieb, M., Monory, K., Lutz, B. & Moreira, F. A. Cannabinoid CB1 receptor in the modulation of stress coping behavior in mice: the role of serotonin and different forebrain neuronal subpopulations. Neuropharmacology 65, 83–89 (2013).
Steiner, M. A., Marsicano, G., Wotjak, C. T. & Lutz, B. Conditional cannabinoid receptor type 1 mutants reveal neuron subpopulation-specific effects on behavioral and neuroendocrine stress responses. Psychoneuroendocrinology 33, 1165–1170 (2008).
Patel, S., Kingsley, P. J., Mackie, K., Marnett, L. J. & Winder, D. G. Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34, 2699–2709 (2009).
Russo, S. J., Murrough, J. W., Han, M. H., Charney, D. S. & Nestler, E. J. Neurobiology of resilience. Nat. Neurosci. 15, 1475–1484 (2012).
Franklin, T. B., Saab, B. J. & Mansuy, I. M. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761 (2012).
Tye, K. M. & Deisseroth, K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci. 13, 251–266 (2012).
Masseck, O. A. et al. Vertebrate cone opsins enable sustained and highly sensitive rapid control of Gi/o signaling in anxiety circuitry. Neuron 81, 1263–1273 (2014).
Soria-Gomez, E. et al. The endocannabinoid system controls food intake via olfactory processes. Nat. Neurosci. 17, 407–415 (2014).
Quarta, C. et al. CB1 signaling in forebrain and sympathetic neurons is a key determinant of endocannabinoid actions on energy balance. Cell. Metab. 11, 273–285 (2010).
Bellocchio, L. et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc. Natl Acad. Sci. USA 110, 4786–4791 (2013).
Araque, A. et al. Gliotransmitters travel in time and space. Neuron 81, 728–739 (2014).
Belanger, M., Allaman, I. & Magistretti, P. J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell. Metab. 14, 724–738 (2011).
Walter, L., Dinh, T. & Stella, N. ATP induces a rapid and pronounced increase in 2-arachidonoylglycerol production by astrocytes, a response limited by monoacylglycerol lipase. J. Neurosci. 24, 8068–8074 (2004).
Stella, N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia 58, 1017–1030 (2010).
Navarrete, M. & Araque, A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron 68, 113–126 (2010).
Min, R. & Nevian, T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat. Neurosci. 15, 746–753 (2012).
Gomez-Gonzalo, M. et al. Endocannabinoids induce lateral long-term potentiation of transmitter release by stimulation of gliotransmission. Cereb. Cortex 25, 3699–3712 (2015).
Ostroff, L. E., Manzur, M. K., Cain, C. K. & LeDoux, J. E. Synapses lacking astrocyte appear in the amygdala during consolidation of Pavlovian threat conditioning. J. Comp. Neurol. 522, 2152–2163 (2014).
Hagemann, T. L., Paylor, R. & Messing, A. Deficits in adult neurogenesis, contextual fear conditioning, and spatial learning in a Gfap mutant mouse model of Alexander disease. J. Neurosci. 33, 18698–18706 (2013).
Clark, D. D. & Sokoloff, L. in Basic Neurochemistry: Molecular, Cellular and Medical Aspects (eds Siegel, G. J. et al.) 637–670 (Lippincott, 1999).
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Phelps, E. A. Emotion and cognition: insights from studies of the human amygdala. Annu. Rev. Psychol. 57, 27–53 (2006).
Rangaraju, V., Calloway, N. & Ryan, T. A. Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014).
Marazziti, D. et al. Mitochondrial alterations and neuropsychiatric disorders. Curr. Med. Chem. 18, 4715–4721 (2011).
Chari-Bitron, A. & Bino, T. Effect of 1-tetrahydrocannabinol on ATPase activity of rat liver mitochondria. Biochem. Pharmacol. 20, 473–475 (1971).
Nunn, A., Guy, G. & Bell, J. D. Endocannabinoids in neuroendopsychology: multiphasic control of mitochondrial function. Phil. Trans. R. Soc. B 367, 3342–3352 (2012).
Zaccagnino, P. et al. The endocannabinoid 2-arachidonoylglicerol decreases calcium induced cytochrome c release from liver mitochondria. J. Bioenerg. Biomembr. 44, 273–280 (2012).
Benard, G. et al. Mitochondrial CB1 receptors regulate neuronal energy metabolism. Nat. Neurosci. 15, 558–564 (2012). This study includes the first description of mitochondrial CB1R and the mechanisms by which it regulates energy metabolism.
Koch, M. et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519, 45–50 (2015).
Morozov, Y. M. et al. Antibodies to cannabinoid type 1 receptor co-react with stomatin-like protein 2 in mouse brain mitochondria. Eur. J. Neurosci. 38, 2341–2348 (2013).
Hebert-Chatelain, E. et al. Cannabinoid control of brain bioenergetics: Exploring the subcellular localization of the CB1 receptor. Mol. Metab. 3, 495–504 (2014).
Bauer, M. et al. Identification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptors. J. Biol. Chem. 287, 36944–36967 (2012).
Straiker, A., Mitjavila, J., Yin, D., Gibson, A. & Mackie, K. Aiming for allosterism: evaluation of allosteric modulators of CB in a neuronal model. Pharmacol. Res. 99, 370–376 (2015).
Hofer, S. C. et al. Localization and production of peptide endocannabinoids in the rodent CNS and adrenal medulla. Neuropharmacology http://dx.doi.org/10.1016/j.neuropharm.2015.03.021 (2015).
Mechoulam, R., Hanus, L. O., Pertwee, R. & Howlett, A. C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 15, 757–764 (2014).
Maccarrone, M., Guzman, M., Mackie, K., Doherty, P. & Harkany, T. Programming of neural cells by (endo)cannabinoids: from physiological rules to emerging therapies. Nat. Rev. Neurosci. 15, 786–801 (2014).
Di Marzo, V., Stella, N. & Zimmer, A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 16, 30–42 (2015).
Van Sickle, M. D. et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332 (2005).
Zhang, H. Y. et al. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc. Natl Acad. Sci. USA 111, 5007–5015 (2014). This study demonstrates the presence of CB2Rs in dopaminergic neurons.
Brusco, A., Tagliaferro, P. A., Saez, T. & Onaivi, E. S. Ultrastructural localization of neuronal brain CB2 cannabinoid receptors. Ann. NY Acad. Sci. 1139, 450–457 (2008).
Dhopeshwarkar, A. & Mackie, K. CB2 cannabinoid receptors as a therapeutic target— What does the future hold? Mol. Pharmacol. 86, 430–437 (2014).
Garcia-Gutierrez, M. S., Garcia-Bueno, B., Zoppi, S., Leza, J. C. & Manzanares, J. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABAA receptors. Br. J. Pharmacol. 165, 951–964 (2012).
Garcia-Gutierrez, M. S. & Manzanares, J. Overexpression of CB2 cannabinoid receptors decreased vulnerability to anxiety and impaired anxiolytic action of alprazolam in mice. J. Psychopharmacol. 25, 111–120 (2011).
Qin, Z. et al. Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron 85, 1319–1331 (2015).
Jung, K. M. et al. Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome. Nat. Commun. 3, 1080 (2012).
Zhang, L. & Alger, B. E. Enhanced endocannabinoid signaling elevates neuronal excitability in fragile X syndrome. J. Neurosci. 30, 5724–5729 (2010).
Tang, A. H. & Alger, B. E. Homer protein-metabotropic glutamate receptor binding regulates endocannabinoid signaling and affects hyperexcitability in a mouse model of fragile X syndrome. J. Neurosci. 35, 3938–3945 (2015).
Dlugos, A., Childs, E., Stuhr, K. L., Hillard, C. J. & de Wit, H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology 37, 2416–2427 (2012).
Blankman, J. L. & Cravatt, B. F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 65, 849–871 (2013).
Rabinak, C. A. et al. Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiol. Learn. Mem. 113, 125–134 (2014).
Roitman, P., Mechoulam, R., Cooper-Kazaz, R. & Shalev, A. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin. Drug Investig. 34, 587–591 (2014).
Puighermanal, E. et al. Dissociation of the pharmacological effects of THC by mTOR blockade. Neuropsychopharmacology 38, 1334–1343 (2013). This study identifies the mammalian target of rapamycin (mTOR) pathway as a novel strategy to dissociate beneficial effects and side effects of cannabinoid agonists.
Hermanson, D. J. et al. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nat. Neurosci. 16, 1291–1298 (2013). This study shows that the inhibition of eicosanoid synthesis by COX2 blockade could represent a novel strategy to tackle anxiety.
Hermanson, D. J., Gamble-George, J. C., Marnett, L. J. & Patel, S. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol. Sci. 35, 358–367 (2014).
Chen, R. et al. Δ9-THC-caused synaptic and memory impairments are mediated through COX-2 signaling. Cell 155, 1154–1165 (2013).
Shore, D. M. et al. Allosteric modulation of a cannabinoid G protein-coupled receptor: binding site elucidation and relationship to G protein signaling. J. Biol. Chem. 289, 5828–5845 (2014).
Ross, R. A. Allosterism and cannabinoid CB1 receptors: the shape of things to come. Trends Pharmacol. Sci. 28, 567–572 (2007).
Vinals, X. et al. Cognitive impairment induced by Δ-9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS. Biol 13, 1002194 (2015). This paper demonstrates that the formation of a heteromer between cannabinoid CB1Rs and serotonin 5-hydroxytryptamine 2A (5-HT2A) receptors is responsible for the memory-impairment effects of cannabis, and that prevention of this interaction could allow selective harnessing of the beneficial effects of cannabinoids without the detrimental effects.
Vallee, M. et al. Pregnenolone can protect the brain from cannabis intoxication. Science 343, 94–98 (2014). This study includes a demonstration of pregnenolone as an endogenous allosteric and signal-specific inhibitor of CB1R that regulates the effect of (endo)cannabinoids on behaviour.
Gibson, H. E., Edwards, J. G., Page, R. S., Van Hook, M. J. & Kauer, J. A. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron 57, 746–759 (2008).
Puente, N. et al. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat. Neurosci. 14, 1542–1547 (2011).
Sigel, E. et al. The major central endocannabinoid directly acts at GABAA receptors. Proc. Natl Acad. Sci. USA 108, 18150–18155 (2011).
Yoshida, T. et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J. Neurosci. 26, 4740–4751 (2006).
Katona, I. et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J. Neurosci. 26, 5628–5637 (2006).
Suarez, J. et al. Distribution of diacylglycerol lipase alpha, an endocannabinoid synthesizing enzyme, in the rat forebrain. Neuroscience 192, 112–131 (2011).
Gao, Y. et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30, 2017–2024 (2010).
Tanimura, A. et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65, 320–327 (2010).
Gulyas, A. I. et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur. J. Neurosci. 20, 441–458 (2004).
Uchigashima, M. et al. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J. Neurosci. 31, 7700–7714 (2011).
Marrs, W. R. et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 13, 951–957 (2010).
Naydenov, A. V. et al. ABHD6 blockade exerts antiepileptic activity in PTZ-induced seizures and in spontaneous seizures in R6/2 mice. Neuron 83, 361–371 (2014).
Viader, A. et al. Metabolic interplay between astrocytes and neurons regulates endocannabinoid action. Cell Rep. 12, 808 (2015).
Nyilas, R. et al. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J. Neurosci. 28, 1058–1063 (2008).
Egertova, M., Simon, G. M., Cravatt, B. F. & Elphick, M. R. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J. Comp. Neurol. 506, 604–615 (2008).
Tsuboi, K. et al. Glycerophosphodiesterase GDE4 as a novel lysophospholipase D: a possible involvement in bioactive N-acylethanolamine biosynthesis. Biochim. Biophys. Acta 1851, 537–548 (2015).
Rahman, I. A., Tsuboi, K., Uyama, T. & Ueda, N. New players in the fatty acyl ethanolamide metabolism. Pharmacol. Res. 86, 1–10 (2014).
Witting, A., Walter, L., Wacker, J., Moller, T. & Stella, N. P2X7 receptors control 2-arachidonoylglycerol production by microglial cells. Proc. Natl Acad. Sci. USA 101, 3214–3219 (2004).
Steindel, F. et al. Neuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampus. J. Neurochem. 124, 795–807 (2013).
Acknowledgements
B.L. was supported by the German Research Foundation (SFB TRR 58, CRC 1080 and FOR 926); G.M. by the Institut national de la santé et de la recherche médicale (INSERM), the European Commission Seventh Framework Programme (REPROBESITY, HEALTH-F2-2008-223713, PAINCAGE and HEALTH-2014-603191), the European Research Council (Endofood, ERC–2010–StG−260515, CannaPreg and ERC-2014-PoC-640923), the Fondation pour la Recherche Medicale (DRM20101220445), the Human Frontiers Science Program, Region Aquitaine, Agence Nationale de la Recherche (ANR Blanc NeuroNutriSens ANR-13-BSV4-0006 and BRAIN ANR-10-LABX-0043); R.M. by the grants SAF2014-59648P, RETICS-RTA#RD12/0028/0023, AGAUR#2014-SGR-1547 and Health-F2-2013-602891; and C.J.H. by the US National Institutes of Health grants DA038663, DA026996 and MH102838.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Endocannabinoid
-
(eCB). A type of lipid signalling molecule derived from arachidonic acid. The eCBs are the endogenous counterparts of the cannabinoids.
- Microglia
-
Immune cells of the brain that are involved in defence.
- Anxiety disorders
-
Mental disorders involving feelings of anxiety and fear, caused by physical or psychological harm. There are different forms, such as general anxiety disorders and specific phobias.
- Thigmotaxis
-
Movement of an organism towards an object (for example, a wall), giving them a sense of increased safety.
- Neophobic behaviour
-
Fear of anything new; unwillingness to try new things and break from routine.
- Polymorphism
-
A genetic variant of a gene, with possible emergence of distinct phenotypes.
- Habituation
-
A form of learning in which an organism reduces its response to a stimulus after repeated presentations of the stimulus.
Rights and permissions
About this article
Cite this article
Lutz, B., Marsicano, G., Maldonado, R. et al. The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16, 705–718 (2015). https://doi.org/10.1038/nrn4036
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn4036
This article is cited by
-
Repeated Δ-9-Tetrahydrocannabinol administration dose dependently increases stablished schedule-induced drinking
Psychopharmacology (2024)
-
Efficacy of cannabinoids in neurodevelopmental and neuropsychiatric disorders among children and adolescents: a systematic review
European Child & Adolescent Psychiatry (2024)
-
Inhibition of fatty acid binding protein-5 in the basolateral amygdala induces anxiolytic effects and accelerates fear memory extinction
Psychopharmacology (2024)
-
Potent dual MAGL/FAAH inhibitor AKU-005 engages endocannabinoids to diminish meningeal nociception implicated in migraine pain
The Journal of Headache and Pain (2023)
-
NAPE-PLD deletion in stress-TRAPed neurons results in an anxiogenic phenotype
Translational Psychiatry (2023)