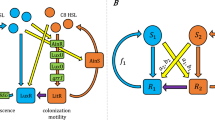
Key Points
-
Understanding why and how bacteria react to the chemical substances that are produced by other bacteria requires an understanding of the nature of the chemical interaction, and the determination of the effect on the fitness of the emitter and receiver.
-
The nature of chemical interactions between bacteria is not necessarily in the form of cooperative signals, but also includes other chemical interactions, such as cues and chemical manipulation.
-
The specificity and reliability of communication depends on the cost of signal production. This cost can be approximated by the biosynthetic cost of signalling, and there is a correlation between the cost of signal production and the specificity of the signalling molecules for the three most common signalling pathways.
-
High relatedness, as occurs, for example, when all cells develop from a single founding cell, is a key factor that promotes cooperation and true communication. Inversely, true communication and cooperation are unlikely to be common between species.
-
A review of the data shows that the evolutionary framework, developed above, allows us to gain a better understanding of quorum sensing and the nature of communication between bacteria, as well as between bacteria and their host.
Abstract
Individual bacteria can alter their behaviour through chemical interactions between organisms in microbial communities ? this is generally referred to as quorum sensing. Frequently, these interactions are interpreted in terms of communication to mediate coordinated, multicellular behaviour. We show that the nature of interactions through quorum-sensing chemicals does not simply involve cooperative signals, but entails other interactions such as cues and chemical manipulations. These signals might have a role in conflicts within and between species. The nature of the chemical interaction is important to take into account when studying why and how bacteria react to the chemical substances that are produced by other bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Greenberg, E. P. Bacterial communication: tiny teamwork. Nature 424, 134 (2003).
Henke, J. M. & Bassler, B. L. Bacterial social engagements. Trends Cell Biol. 14, 648?656 (2004).
Kolenbrander, P. E., Egland, P. G., Diaz, P. I. & Palmer, R. J. Jr. Genome?genome interactions: bacterial communities in initial dental plaque. Trends Microbiol. 13, 11?15 (2005).
Lyon, G. J. & Novick, R. P. Peptide signaling in Staphylococcus aureus and other Gram-positive bacteria. Peptides 25, 1389?1403 (2004).
Parsek, M. R. & Greenberg, E. P. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 13, 27?33 (2005).
Crespi, B. J. The evolution of social behavior in microorganisms. Trends Ecol. Evol. 16, 178?183 (2001).
Bourke, A. F. G. & Franks, N. R. Social Evolution in Ants (Princeton University Press, Princeton, 1995).
Hamilton, W. D. The genetical evolution of social behaviour. I. J. Theor. Biol. 7, 1?16 (1964).
Hamilton, W. D. The genetical evolution of social behaviour. II. J. Theor. Biol. 7, 17?52 (1964).
Keller, L. Levels of Selection in Evolution (Princeton University Press, Princeton 1999). A book that reviews examples of conflicts that occur at the different levels of biological organization (for example, between genes within organisms and between organisms within social groups).
Szathmary, E. & Maynard Smith, J. The Major Transitions in Evolution (Freeman Press, Oxford 1995).
Keller, L. & Reeve, H. K. Kin selection. In Encyclopedia of Evolution 595?600 (Oxford University Press, Oxford, 2002).
Queller, D. C. Does population viscosity promote kin selection? Trends Ecol. Evol. 7, 322?324 (1992).
Redfield, R. J. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10, 365?370 (2002). A thought-provoking commentary that challenges the conventional view of quorum sensing.
Shompole, S. et al. Biphasic intracellular expression of Staphylococcus aureus virulence factors and evidence for Agr-mediated diffusion sensing. Mol. Microbiol. 49, 919?927 (2003).
McFall-Ngai, M. J. Negotiations between animals and bacteria: the 'diplomacy' of the squid?Vibrio symbiosis. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 126, 471?480 (2000).
Ruby, E. G. The Euprymna scolopes?Vibrio fischeri symbiosis: a biomedical model for the study of bacterial colonization of animal tissue. J. Mol. Microbiol. Biotechnol. 1, 13?21 (1999).
Visick, K. L. Layers of signaling in a bacterium?host association. J. Bacteriol. 187, 3603?3606 (2005).
Hastings, J. W. & Greenberg, E. P. Quorum sensing: the explanation of a curious phenomenon reveals a common characteristic of bacteria. J. Bacteriol. 181, 2667?2668 (1999).
Sachs, J. L., Mueller, U. G., Wilcox, T. P. & Bull, J. J. The evolution of cooperation. Q. Rev. Biol. 79, 135?160 (2004). A review of the mechanisms and selective forces that can maintain cooperation within and between species.
Manefield, M. & Turner, S. L. Quorum sensing in context: out of molecular biology and into microbial ecology. Microbiology 148, 3762?3764 (2002).
Kleerebezem, M., Quadri, L. E., Kuipers, O. P. & de Vos, W. M. Quorum sensing by peptide pheromones and two-component signal-transduction systems in Gram-positive bacteria. Mol. Microbiol. 24, 895?904 (1997).
Mayville, P. et al. Structure?activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc. Natl Acad. Sci. USA 96, 1218?1223 (1999).
Ji, G., Beavis, R. & Novick, R. P. Bacterial interference caused by autoinducing peptide variants. Science 276, 2027?2030 (1997).
Lyon, G. J., Wright, J. S., Muir, T. W. & Novick, R. P. Key determinants of receptor activation in the agr autoinducing peptides of Staphylococcus aureus. Biochemistry 41, 10095?10104 (2002).
Eberl, L. N-acyl homoserinelactone-mediated gene regulation in Gram-negative bacteria. Syst. Appl. Microbiol. 22, 493?506 (1999).
Fuqua, C., Parsek, M. R. & Greenberg, E. P. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35, 439?468 (2001).
Pearson, J. P. et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl Acad. Sci. USA 91, 197?201 (1994).
Winson, M. K. et al. Multiple N-acyl-L-homoserine lactone signal molecules regulate production of virulence determinants and secondary metabolites in Pseudomonas aeruginosa. Proc. Natl Acad. Sci. USA 92, 9427?9431 (1995).
Shaw, P. D. et al. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl Acad. Sci. USA 94, 6036?6041 (1997).
Surette, M. G., Miller, M. B. & Bassler, B. L. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl Acad. Sci. USA 96, 1639?1644 (1999).
Xavier, K. B. & Bassler, B. L. LuxS quorum sensing: more than just a numbers game. Curr. Opin. Microbiol. 6, 191?197 (2003).
Vendeville, A., Winzer, K., Heurlier, K., Tang, C. M. & Hardie, K. R. Making 'sense' of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nature Rev. Microbiol. 3, 383?396 (2005).
Sun, J., Daniel, R., Wagner-Dobler, I. & Zeng, A. P. Is autoinducer-2 a universal signal for interspecies communication: a comparative genomic and phylogenetic analysis of the synthesis and signal transduction pathways. BMC Evol. Biol. 4, 36 (2004).
Schauder, S., Shokat, K., Surette, M. G. & Bassler, B. L. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41, 463?476 (2001).
Burgess, N. A. et al. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology 148, 763?772 (2002).
Chen, X. et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415, 545?549 (2002). This paper reports the structure of AI-2 as it is bound in the Vibrio harveyi receptor LuxP complexed with boron (see also reference 41).
Miller, S. T. et al. Salmonella typhimurium recognizes a chemically distinct form of the bacterial quorum-sensing signal AI-2. Mol. Cell 15, 677?687 (2004).
Winzer, K., Hardie, K. R. & Williams, P. LuxS and autoinducer-2: their contribution to quorum sensing and metabolism in bacteria. Adv. Appl. Microbiol. 53, 291?396 (2003).
Travisano, M. & Velicer, G. J. Strategies of microbial cheater control. Trends Microbiol. 12, 72?78 (2004).
Velicer, G. J. Social strife in the microbial world. Trends Microbiol. 11, 330?337 (2003). This review provides examples of selfish strategies that undermine cooperative behaviour across various microbial systems.
Maynard Smith, J. & Harper, D. Animal Signals (Oxford University Press, Oxford, 2003). A book that discusses, from a theoretical perspective, the problem of communication in animals.
Duan, K., Dammel, C., Stein, J., Rabin, H. & Surette, M. G. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50, 1477?1491 (2003). Demonstrates the interaction of oropharyngeal flora with P. aeruginosa , which results in enhanced pathogenicity. This is an example of the pathogen using AI-2 that is produced by other bacteria as a cue.
Varaldi, J. et al. Infectious behavior in a parasitoid. Science 302, 1930 (2003).
Thomas, F., Adamo, S. & Moore, J. Parasitic manipulation: where are we and where should we go? Behav. Processes 68, 185?199 (2005).
Werren, J. H. Biology of Wolbachia. Annu. Rev. Entomol. 42, 587?609 (1997).
Kolenbrander, P. E. et al. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 66, 486?505 (2002).
Marsh, P. D. Dental plaque as a microbial biofilm. Caries Res. 38, 204?211 (2004).
Egland, P. G., Palmer, R. J. Jr & Kolenbrander, P. E. Interspecies communication in Streptococcus gordonii?Veillonella atypica biofilms: signaling in flow conditions requires juxtaposition. Proc. Natl Acad. Sci. USA 101, 16917?16922 (2004). Demonstrates an example of chemical manipulation between two oral bacteria, in which one strain produces a signal that alters the metabolism of the other. It also demonstrates that signalling occurs only on a very local scale.
Griffin, A. S., West, S. A. & Buckling, A. Cooperation and competition in pathogenic bacteria. Nature 430, 1024?1027 (2004). Shows that experimental manipulation of relatedness affects the level cooperative siderophore production in P. aeruginosa .
Fiegna, F. & Velicer, G. J. Exploitative and hierarchical antagonism in a cooperative bacteria. PLoS Biol. 3, 1980?1087 (2005).
Escalante, R. & Vicente, J. J. Dictyostelium discoideum: a model system for differentiation and patterning. Int. J. Dev. Biol. 44, 819?835 (2000).
Weijer, C. J. Dictyostelium morphogenesis. Curr. Opin. Genet. Dev. 14, 392?398 (2004).
Kaiser, D. Signaling in myxobacteria. Annu. Rev. Microbiol. 58, 75?98 (2004).
Kaplan, H. B. Multicellular development and gliding motility in Myxococcus xanthus. Curr. Opin. Microbiol. 6, 572?577 (2003).
Sogaard-Andersen, L. Cell polarity, intercellular signalling and morphogenetic cell movements in Myxococcus xanthus. Curr. Opin. Microbiol. 7, 587?593 (2004).
Shimkets, L. J. Intercellular signaling during fruiting-body development of Myxococcus xanthus. Annu. Rev. Microbiol. 53, 525?549 (1999).
Jelsbak, L. & Sogaard-Andersen, L. The cell surface-associated intercellular C-signal induces behavioral changes in individual Myxococcus xanthus cells during fruiting body morphogenesis. Proc. Natl Acad. Sci. USA 96, 5031?5036 (1999).
Kruse, T., Lobedanz, S., Berthelsen, N. M. & Sogaard-Andersen, L. C-signal: a cell surface-associated morphogen that induces and co-ordinates multicellular fruiting body morphogenesis and sporulation in Myxococcus xanthus. Mol. Microbiol. 40, 156?168 (2001).
Strassmann, J. E. Bacterial cheaters. Nature 404, 555?556 (2000).
Velicer, G. J., Kroos, L. & Lenski, R. E. Developmental cheating in the social bacterium Myxococcus xanthus. Nature 404, 598?601 (2000). Demonstrates that some mutants that are defective for fruiting-body development are over-represented among the spores that are produced. This shows a trade-off between cooperative traits that is beneficial to the group but costly to the individual.
Chandler, J. R. & Dunny, G. M. Enterococcal peptide sex pheromones: synthesis and control of biological activity. Peptides 25, 1377?1388 (2004).
Dunny, G. M., Antiporta, M. H. & Hirt, H. Peptide pheromone-induced transfer of plasmid pCF10 in Enterococcus faecalis: probing the genetic and molecular basis for specificity of the pheromone response. Peptides 22, 1529?1539 (2001).
Diaz, E. Bacterial degradation of aromatic pollutants: a paradigm of metabolic versatility. Int. Microbiol. 7, 173?180 (2004).
Lewenza, S., Visser, M. B. & Sokol, P. A. Interspecies communication between Burkholderia cepacia and Pseudomonas aeruginosa. Can. J. Microbiol. 48, 707?716 (2002).
Riedel, K. et al. N-acylhomoserine-lactone-mediated communication between Pseudomonas aeruginosa and Burkholderia cepacia in mixed biofilms. Microbiology 147, 3249?3262 (2001).
Chambers, C. E., Visser, M. B., Schwab, U. & Sokol, P. A. Identification of N-acylhomoserine lactones in mucopurulent respiratory secretions from cystic fibrosis patients. FEMS Microbiol. Lett. 244, 297?304 (2005).
Geisenberger, O. et al. Production of N-acyl-L-homoserine lactones by P. aeruginosa isolates from chronic lung infections associated with cystic fibrosis. FEMS Microbiol. Lett. 184, 273?278 (2000).
Romano, J. D. & Kolter, R. Pseudomonas?Saccharomyces interactions: influence of fungal metabolism on bacterial physiology and survival. J. Bacteriol. 187, 940?948 (2005).
DeLoney-Marino, C. R., Wolfe, A. J. & Visick, K. L. Chemoattraction of Vibrio fischeri to serine, nucleosides, and N-acetylneuraminic acid, a component of squid light-organ mucus. Appl. Environ. Microbiol. 69, 7527?7530 (2003).
Graf, J., Dunlap, P. V. & Ruby, E. G. Effect of transposon-induced motility mutations on colonization of the host light organ by Vibrio fischeri. J. Bacteriol. 176, 6986?6991 (1994).
Millikan, D. S. & Ruby, E. G. Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects. Appl. Environ. Microbiol. 68, 2519?2528 (2002).
Lupp, C. & Ruby, E. G. Vibrio fischeri uses two quorum-sensing systems for the regulation of early and late colonization factors. J. Bacteriol. 187, 3620?3629 (2005).
Lupp, C. & Ruby, E. G. Vibrio fischeri LuxS and AinS: comparative study of two signal synthases. J. Bacteriol. 186, 3873?3881 (2004).
Doino, J. & McFall-Ngai, M. transient exposure to symbiosis-competent bacteria induces light organ morphogenesis in the host squid. Biol. Bull. 189, 347?355 (1995).
Doino Lemus, J. & McFall-Ngai, M. J. Alterations in the proteome of the Euprymna scolopes light organ in response to symbiotic Vibrio fischeri. Appl. Environ. Microbiol. 66, 4091?4097 (2000).
Foster, J. S., Apicella, M. A. & McFall-Ngai, M. J. Vibrio fischeri lipopolysaccharide induces developmental apoptosis, but not complete morphogenesis, of the Euprymna scolopes symbiotic light organ. Dev. Biol. 226, 242?254 (2000).
Visick, K. L., Foster, J., Doino, J., McFall-Ngai, M. & Ruby, E. G. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 182, 4578?4586 (2000).
Ruby, E. G. & McFall-Ngai, M. J. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 7, 414?420 (1999).
Kiers, E. T., Rousseau, R. A., West, S. A. & Denison, R. F. Host sanctions and the legume?Rhizobium mutualism. Nature 425, 78?81 (2003).
Li, L., Hooi, D., Chhabra, S. R., Pritchard, D. & Shaw, P. E. Bacterial N-acylhomoserine lactone-induced apoptosis in breast carcinoma cells correlated with down-modulation of STAT3. Oncogene 23, 4894?4902 (2004).
Tateda, K. et al. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 71, 5785?5793 (2003).
Ritchie, A. J. et al. The Pseudomonas aeruginosa quorum-sensing molecule N-3-(oxododecanoyl)-L-homoserine lactone inhibits T-cell differentiation and cytokine production by a mechanism involving an early step in T-cell activation. Infect. Immun. 73, 1648?1655 (2005).
Smith, R. S., Kelly, R., Iglewski, B. H. & Phipps, R. P. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclooxygenase-2 and prostaglandin E2 production in human lung fibroblasts: implications for inflammation. J. Immunol. 169, 2636?2642 (2002).
Telford, G. et al. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone has immunomodulatory activity. Infect. Immun. 66, 36?42 (1998).
Hooi, D. S., Bycroft, B. W., Chhabra, S. R., Williams, P. & Pritchard, D. I. Differential immune modulatory activity of Pseudomonas aeruginosa quorum-sensing signal molecules. Infect. Immun. 72, 6463?6470 (2004).
Chun, C. K., Ozer, E. A., Welsh, M. J., Zabner, J. & Greenberg, E. P. Inactivation of a Pseudomonas aeruginosa quorum-sensing signal by human airway epithelia. Proc. Natl Acad. Sci. USA 101, 3587?3590 (2004).
Givskov, M. et al. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 178, 6618?6622 (1996).
Manefield, M. et al. Evidence that halogenated furanones from Delisea pulchra inhibit acylated homoserine lactone (AHL)-mediated gene expression by displacing the AHL signal from its receptor protein. Microbiology 145, 283?291 (1999).
Solomon, J. M., Lazazzera, B. A. & Grossman, A. D. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10, 2014?2024 (1996).
Tummler, B. et al. Infections with Pseudomonas aeruginosa in patients with cystic fibrosis. Behring Inst. Mitt. 98, 249?255 (1997).
Schaber, J. A. et al. Analysis of quorum sensing-deficient clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 53, 841?853 (2004).
Blehert, D. S., Palmer, R. J. Jr, Xavier, J. B., Almeida, J. S. & Kolenbrander, P. E. Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions. J. Bacteriol. 185, 4851?4860 (2003).
McNab, R. et al. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185, 274?284 (2003).
Li, Y. H., Lau, P. C., Lee, J. H., Ellen, R. P. & Cvitkovitch, D. G. Natural genetic transformation of Streptococcus mutans growing in biofilms. J. Bacteriol. 183, 897?908 (2001).
Li, Y. H. et al. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. J. Bacteriol. 184, 2699?2708 (2002).
Acknowledgements
We thank B. Bassler, C. Davidson, P. Greenberg, G. Velicer, K. Visick, P. Williams and four reviewers for their useful comments on the manuscript. The authors were supported by grants from the Swiss National Science Foundation (L.K.) and the Canadian Institutes of Health Research (M.G.S.).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome Project
Salmonella enterica serovar Typhimurium
FURTHER INFORMATION
Glossary
- Secondary metabolite
-
Chemical produced by the cell that is not essential for maintenance of cellular function or for normal growth of the organism.
- Quorum sensing
-
A system by which bacteria communicate and obtain information on bacterial density in their environment.
- Autoinducer
-
A system by which bacteria communicate. Signalling molecules are chemicals, similar to pheromones that are produced by an individual bacterium, which can affect the behaviour of surrounding bacteria.
- Signal
-
Any act, structure or chemical emission that alters the behaviour and gene expression of other organisms which evolved because of that effect, and that is effective because the receiver's response has also evolved.
- N-acyl homoserine lactone
-
A group of intercellular signalling molecules, produced by some Gram-negative bacteria, made up of a homoserinelactone ring with an N-linked acyl side chain.
- LuxS/autoinducer-2
-
(AI-2). Refers to a conserved gene (luxS) found in both Gram-negative and Gram-positive bacteria that mediates production of a common signalling molecule autoinducer-2.
- Pre-protein
-
A protein initially synthesized as an inactive form that undergoes proteolytic cleavage to release the active protein or peptide.
- Chemical manipulation
-
Chemical emission that alters the behaviour and gene expression of other organisms. However, contrary to a signal, the effect induced by chemical manipulation has a negative effect on the fitness of the receiver.
- Cue
-
An act, structure or chemical emission that alters the behaviour and gene expression of other organisms. However, contrary to a signal, it did not evolve specifically for that effect.
- Fruiting body
-
A specialized macroscopic, spore-producing structure that is formed by some fungi (for example, mushrooms), slime moulds and mycobacteria.
- Cheater
-
An individual obtaining benefits from a collectively produced public good that are disproportionally large relative to its own contribution to that good.
- Conjugative plasmid
-
A plasmid that can move from one cell to another during the process of conjugation.
- Symbiont
-
An organism living in a close, long-lasting and more or less beneficial association with another organism.
- Syntrophic association
-
A process whereby two or more microorganisms cooperate to degrade a substrate or substrates that neither can degrade alone.
- Niche partitioning
-
The division of a limiting resource(s) among the species that share it.
- Flushing
-
The removal of chemicals and/or individuals from an area by a current or flow through the area.
Rights and permissions
About this article
Cite this article
Keller, L., Surette, M. Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4, 249–258 (2006). https://doi.org/10.1038/nrmicro1383
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1383
This article is cited by
-
Impact of gut microbiota composition on black cutworm, Agrotis ipsilon (hufnagel) metabolic indices and pesticide degradation
Animal Microbiome (2023)
-
The evolution of mechanisms to produce phenotypic heterogeneity in microorganisms
Nature Communications (2022)
-
Circuit-guided population acclimation of a synthetic microbial consortium for improved biochemical production
Nature Communications (2022)
-
Biofilm as an adaptation strategy to extreme conditions
Rendiconti Lincei. Scienze Fisiche e Naturali (2022)
-
Polymers, the Light at the End of Dark Fermentation: Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by a Photoheterotrophic Consortium
Journal of Polymers and the Environment (2022)