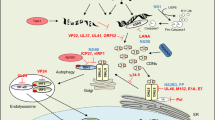
Key Points
-
Bacteria and viruses that interact directly with the host cell cytosol risk triggering pro-inflammatory caspase-dependent defences, which include pyroptosis, lytic cell death and the secretion of the pro-inflammatory cytokines interleukin-1β (IL-1β) and IL-18.
-
Caspase 1 can be activated by receptors that detect specific pathogen-derived molecules (such as flagellin, type III secretion components or certain nucleic acids), or by the production and incorrect localization of host-derived molecules. Caspase 11, caspase 4 and caspase 5 are pro-inflammatory caspases that trigger pyroptosis in response to cytosolic lipopolysaccharide.
-
Receptor engagement initiates the oligomerization of receptors, adaptors and caspase 1 into a multiprotein complex termed the inflammasome, in which the activation of caspase 1 and the cleavage of pro-IL-1β and pro-IL-18 occur.
-
Inflammation is a threshold-driven process. Extracellular and endosomal detection of pathogen-derived molecules can activate the transcription of inflammasome components, which 'primes' the cell for the activation of pro-inflammatory caspases if intracellular triggers are subsequently detected.
-
Pathogens have evolved strategies to deal with inflammation. Some bacteria evade priming or the activation of pro-inflammatory caspases by downregulating or modifying inflammatory triggers. Some bacteria and viruses directly interfere with the assembly of the inflammasome, the enzymatic activity of caspase-1, or pro-inflammatory cytokine signalling. Yersinia pestis, the aetiological agent of plague, is an example of a pathogen that uses several strategies to delay inflammation.
Abstract
Pathogens have evolved to complete the virulence cycle of colonization, replication and dissemination in intimate association with a complex network of extracellular and intracellular surveillance systems that guard tissue spaces. In this Review, we discuss the strategies used by bacteria and viruses to evade or inhibit intracellular detection that is coupled to pro-inflammatory caspase-dependent protective responses. Such strategies include alterations of lipopolysaccharide (LPS) structures, the regulated expression of components of type III secretion systems, and the utilization of proteins that inhibit inflammasome formation, the enzymatic activity of caspases and cytokine signalling. Inflammation is crucial in response to exposure to pathogens, but is potentially damaging and thus tightly regulated. The threshold for the activation of pro-inflammatory caspases is determined by the immediate stimulus in the context of previous signals. Pathogen, genetic and situational factors modulate this threshold, which determines the ability of the host to resist infection while minimizing harm.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Nathan, C. Points of control in inflammation. Nature 420, 846–852 (2002).
Brubaker, S. W., Bonham, K. S., Zanoni, I. & Kagan, J. C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290 (2015).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
von Moltke, J., Ayres, J. S., Kofoed, E. M., Chavarría-Smith, J. & Vance, R. E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 31, 73–106 (2013).
Kanneganti, T. D. Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 (2010).
Andrews-Polymenis, H. L., Bäumler, A. J., McCormick, B. A. & Fang, F. C. Taming the elephant: Salmonella biology, pathogenesis, and prevention. Infect. Immun. 78, 2356–2369 (2010).
Ogawa, M., Handa, Y., Ashida, H., Suzuki, M. & Sasakawa, C. The versatility of Shigella effectors. Nat. Rev. Microbiol. 6, 11–16 (2008).
Navarro, L., Alto, N. M. & Dixon, J. E. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 8, 21–27 (2005).
Isaac, D. T. & Isberg, R. Master manipulators: an update on Legionella pneumophila Icm/Dot translocated substrates and their host targets. Future Microbiol. 9, 343–359 (2014).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Wilkins, C. & Gale, M. Recognition of viruses by cytoplasmic sensors. Curr. Opin. Immunol. 22, 41–47 (2010).
Cummings, L. A., Barrett, S. L., Wilkerson, W. D., Fellnerova, I. & Cookson, B. T. FliC-specific CD4+ T cell responses are restricted by bacterial regulation of antigen expression. J. Immunol. 174, 7929–7938 (2005).
LaRock, D. L., Chaudhary, A. & Miller, S. I. Salmonellae interactions with host processes. Nat. Rev. Microbiol. 13, 191–205 (2015).
Rebeil, R., Ernst, R. K., Gowen, B. B., Miller, S. I. & Hinnebusch, B. J. Variation in lipid A structure in the pathogenic yersiniae. Mol. Microbiol. 52, 1363–1373 (2004).
Larock, C. N. & Cookson, B. T. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12, 799–805 (2012). This study is the first account of a bacterial effector protein that inhibits the activation of caspase 1.
Kobayashi, T. et al. The Shigella OspC3 effector inhibits caspase-4, antagonizes inflammatory cell death, and promotes epithelial infection. Cell Host Microbe 13, 570–583 (2013).
Ray, C. A. et al. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 69, 597–604 (1992). This study is the first account of a viral protein that inhibits the activation of caspase 1.
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002). This paper provides the first description of the inflammasome.
Ting, J. P. et al. The NLR gene family: a standard nomenclature. Immunity 28, 285–287 (2008).
Kofoed, E. M. & Vance, R. E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 (2011). References 20 and 21 demonstrate the requirement for NAIPs to activate the NLRC4 inflammasome, and the ligand specificities for NAIP2, NAIP5, NAIP6 and human NAIP.
Zhao, Y. et al. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 (2011).
Rayamajhi, M., Zak, D. E., Chavarria-Smith, J., Vance, R. E. & Miao, E. A. Cutting edge: mouse NAIP1 detects the type III secretion system needle protein. J. Immunol. 191, 3986–3989 (2013).
Kortmann, J., Brubaker, S. W. & Monack, D. M. Cutting edge: inflammasome activation in primary human macrophages is dependent on flagellin. J. Immunol. 195, 815–819 (2015).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Lu, A. & Wu, H. Structural mechanisms of inflammasome assembly. FEBS J. 282, 435–444 (2015).
Nieva, J. L., Madan, V. & Carrasco, L. Viroporins: structure and biological functions. Nat. Rev. Microbiol. 10, 563–574 (2012).
Fink, S. L., Bergsbaken, T. & Cookson, B. T. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proc. Natl Acad. Sci. USA 105, 4312–4317 (2008). This study demonstrates a common effector pathway that leads to pyroptosis downstream from distinct stimuli and details the first microscopic observation of activated caspase 1 organized into inflammasome foci in the cytoplasm of pyroptotic cells.
Boyden, E. D. & Dietrich, W. F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Levinsohn, J. L. et al. Anthrax lethal factor cleavage of NLRP1 is required for activation of the inflammasome. PLoS Pathog. 8, e1002638 (2012).
Cirelli, K. M. et al. Inflammasome sensor NLRP1 controls rat macrophage susceptibility to Toxoplasma gondii. PLoS Pathog. 10, e1003927 (2014).
Bruey, J. M. et al. Bcl-2 and Bcl-XL regulate pro-inflammatory caspase-1 activation by interaction with NALP1. Cell 129, 45–56 (2007).
Van Opdenbosch, N. et al. Activation of the NLRP1b inflammasome independently of ASC-mediated caspase-1 autoproteolysis and speck formation. Nat. Commun. 5, 3209 (2014).
Guey, B., Bodnar, M., Manié, S. N., Tardivel, A. & Petrilli, V. Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function. Proc. Natl Acad. Sci. USA 111, 17254–17259 (2014).
Finger, J. N. et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J. Biol. Chem. 287, 25030–25037 (2012).
Pothlichet, J. et al. Type I IFN triggers RIG-I/TLR3/NLRP3-dependent inflammasome activation in influenza A virus infected cells. PLoS Pathog. 9, e1003256 (2013).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Needham, B. D. & Trent, M. S. Fortifying the barrier: the impact of lipid A remodelling on bacterial pathogenesis. Nat. Rev. Microbiol. 11, 467–481 (2013).
Hagar, J. A., Powell, D. A., Aachoui, Y., Ernst, R. K. & Miao, E. A. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341, 1250–1253 (2013). References 39 and 40 show that cytoplasmic LPS from bacteria that escape the vacuole activates caspase 11-dependent pyroptosis.
Aachoui, Y. et al. Caspase-11 protects against bacteria that escape the vacuole. Science 339, 975–978 (2013).
Knodler, L. A. et al. Non-canonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014).
Shi, J. et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192 (2014).
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein- and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). This study demonstrates that activated caspase 11 causes pyroptosis, but requires caspase 1, NLRP3 and ASC for the production of mature IL-1β.
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
Meunier, E. et al. Caspase-11 activation requires lysis of pathogen-containing vacuoles by IFN-induced GTPases. Nature 509, 366–370 (2014).
Barton, G. M. & Kagan, J. C. A cell biological view of Toll-like receptor function: regulation through compartmentalization. Nat. Rev. Immunol. 9, 535–542 (2009).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009). This study demonstrates the priming (transcriptional upregulation) of the NLRP3 inflammasome through the NFκB pathway.
Henry, T., Brotcke, A., Weiss, D. S., Thompson, L. J. & Monack, D. M. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J. Exp. Med. 204, 987–994 (2007).
DeYoung, K. L. et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 15, 453–457 (1997).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Sims, J. E. & Smith, D. E. The IL-1 family: regulators of immunity. Nat. Rev. Immunol. 10, 89–102 (2010).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Dinarello, C. A., Simon, A. & van der Meer, J. W. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 (2012).
Kawahara, K., Tsukano, H., Watanabe, H., Lindner, B. & Matsuura, M. Modification of the structure and activity of lipid A in Yersinia pestis lipopolysaccharide by growth temperature. Infect. Immun. 70, 4092–4098 (2002).
Montminy, S. W. et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 7, 1066–1073 (2006).
Vladimer, G. I. et al. The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37, 96–107 (2012).
Li, Y. et al. LPS remodeling is an evolved survival strategy for bacteria. Proc. Natl Acad. Sci. USA 109, 8716–8721 (2012).
de Jong, M. F., Rolán, H. G. & Tsolis, R. M. Innate immune encounters of the (Type) 4th kind: Brucella. Cell. Microbiol. 12, 1195–1202 (2010).
Toman, R. et al. Physicochemical characterization of the endotoxins from Coxiella burnetii strain Priscilla in relation to their bioactivities. BMC Biochem. 5, 1 (2004).
Semper, R. P. et al. Helicobacter pylori-induced IL-1β secretion in innate immune cells is regulated by the NLRP3 inflammasome and requires the Cag pathogenicity island. J. Immunol. 193, 3566–3576 (2014).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Kim, D. J., Park, J. H., Franchi, L., Backert, S. & Núñez, G. The Cag pathogenicity island and interaction between TLR2/NOD2 and NLRP3 regulate IL-1β production in Helicobacter pylori infected dendritic cells. Eur. J. Immunol. 43, 2650–2658 (2013).
Dotson, R. J. et al. Repression of inflammasome by Francisella tularensis during early stages of infection. J. Biol. Chem. 288, 23844–23857 (2013).
Andersen-Nissen, E. et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc. Natl Acad. Sci. USA 102, 9247–9252 (2005).
Terwagne, M. et al. Innate immune recognition of flagellin limits systemic persistence of Brucella. Cell. Microbiol. 15, 942–960 (2013).
Stecher, B. et al. Motility allows S. Typhimurium to benefit from the mucosal defence. Cell. Microbiol. 10, 1166–1180 (2008). References 69, 75 and 76 demonstrate the specific mechanisms that enable S . Typhimurium to benefit from the environment in the inflamed gut.
Galán, J. E. & Curtiss, R. Cloning and molecular characterization of genes whose products allow Salmonella Typhimurium to penetrate tissue culture cells. Proc. Natl Acad. Sci. USA 86, 6383–6387 (1989).
Cummings, L. A., Wilkerson, W. D., Bergsbaken, T. & Cookson, B. T. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61, 795–809 (2006). This study demonstrates the heterogeneous expression of flagellar genes in Salmonella spp. both in vitro and in vivo , and the downregulation of fliC at systemic sites during infection.
Stewart, M. K., Cummings, L. A., Johnson, M. L., Berezow, A. B. & Cookson, B. T. Regulation of phenotypic heterogeneity permits Salmonella evasion of the host caspase-1 inflammatory response. Proc. Natl Acad. Sci. USA 108, 20742–20747 (2011). This study demonstrates that the control of heterogeneous flagellar gene expression in Salmonella spp. enables these species to evade the activation of caspase 1 in systemic tissues during infection.
Stewart, M. K. & Cookson, B. T. Mutually repressing repressor functions and multi-layered cellular heterogeneity regulate the bistable Salmonella fliC census. Mol. Microbiol. 94, 1272–1284 (2014).
Iyoda, S., Kamidoi, T., Hirose, K., Kutsukake, K. & Watanabe, H. A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb. Pathog. 30, 81–90 (2001).
Thiennimitr, P. et al. Intestinal inflammation allows Salmonella to use ethanolamine to compete with the microbiota. Proc. Natl Acad. Sci. USA 108, 17480–17485 (2011).
Winter, S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010).
Laughlin, R. C. et al. Spatial segregation of virulence gene expression during acute enteric infection with Salmonella enterica serovar Typhimurium. mBio 5, e00946-13 (2014).
Miao, E. A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin-1β via Ipaf. Nat. Immunol. 7, 569–575 (2006). This study shows that the Salmonella -induced caspase 1-dependent pyroptosis of macrophages is mediated by cytosolic flagellin and requires NLRC4.
Miao, E. A. et al. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc. Natl Acad. Sci. USA 107, 3076–3080 (2010). This study finds that the SPI-1 T3SS rod protein PrgJ in Salmonella spp., but not the SPI-2 T3SS rod protein SsaI, activates caspase 1 in an NLRC4-dependent manner; full virulence requires that SsaI not be detected by NLRC4. The authors identify a specific rod protein amino acid motif that correlates with the activation of caspase 1.
Brennan, M. A. & Cookson, B. T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000). The first mechanistic description showing that caspase 1-dependent macrophage cell death, later termed pyroptosis, is readily distinguishable from apoptosis.
Fass, E. & Groisman, E. A. Control of Salmonella pathogenicity island-2 gene expression. Curr. Opin. Microbiol. 12, 199–204 (2009).
Dalebroux, Z. D. & Miller, S. I. Salmonellae PhoPQ regulation of the outer membrane to resist innate immunity. Curr. Opin. Microbiol. 17, 106–113 (2014).
Miao, E. A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Pujol, C. & Bliska, J. B. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 71, 5892–5899 (2003).
Parkhill, J. et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413, 523–527 (2001).
Kapatral, V., Olson, J. W., Pepe, J. C., Miller, V. L. & Minnich, S. A. Temperature-dependent regulation of Yersinia enterocolitica Class III flagellar genes. Mol. Microbiol. 19, 1061–1071 (1996).
Chen, T. H. & Elberg, S. S. Scanning electron microscopic study of virulent Yersinia pestis and Yersinia pseudotuberculosis type 1. Infect. Immun. 15, 972–977 (1977).
Brodsky, I. E. et al. A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7, 376–387 (2010).
Dewoody, R. S., Merritt, P. M. & Marketon, M. M. Regulation of the Yersinia type III secretion system: traffic control. Front. Cell. Infect. Microbiol. 3, 4 (2013).
Zwack, E. E. et al. Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. mBio 6, e02095-14 (2015).
Lee, S. H., Stehlik, C. & Reed, J. C. Cop, a caspase recruitment domain-containing protein and inhibitor of caspase-1 activation processing. J. Biol. Chem. 276, 34495–34500 (2001).
Druilhe, A., Srinivasula, S. M., Razmara, M., Ahmad, M. & Alnemri, E. S. Regulation of IL-1β generation by Pseudo-ICE and ICEBERG, two dominant negative caspase recruitment domain proteins. Cell Death Differ. 8, 649–657 (2001).
Atianand, M. K. & Harton, J. A. Uncoupling of Pyrin-only protein 2 (POP2)-mediated dual regulation of NF-κB and the inflammasome. J. Biol. Chem. 286, 40536–40547 (2011).
Annand, R. R. et al. Caspase-1 (interleukin-1β-converting enzyme) is inhibited by the human serpin analogue proteinase inhibitor 9. Biochem. J. 342, 655–665 (1999).
Li, J., Yin, H. L. & Yuan, J. Flightless-I regulates pro-inflammatory caspases by selectively modulating intracellular localization and caspase activity. J. Cell Biol. 181, 321–333 (2008).
Dinarello, C. A., Novick, D., Kim, S. & Kaplanski, G. Interleukin-18 and IL-18 binding protein. Front. Immunol. 4, 289 (2013).
Sutterwala, F. S. et al. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J. Exp. Med. 204, 3235–3245 (2007).
Cunha, L. D. et al. Inhibition of inflammasome activation by Coxiella burnetii type IV secretion system effector IcaA. Nat. Commun. 6, 10205 (2015).
Dorfleutner, A. et al. A Shope fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35, 685–694 (2007).
Johnston, J. B. et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598 (2005).
Rahman, M. M. & McFadden, G. Myxoma virus lacking the pyrin-like protein M013 is sensed in human myeloid cells by both NLRP3 and multiple Toll-like receptors, which independently activate the inflammasome and NF-κB innate response pathways. J. Virol. 85, 12505–12517 (2011).
Silverman, G. A. et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 276, 33293–33296 (2001).
Garcia-Calvo, M. et al. Inhibition of human caspases by peptide-based and macromolecular inhibitors. J. Biol. Chem. 273, 32608–32613 (1998).
Haller, S. L., Peng, C., McFadden, G. & Rothenburg, S. Poxviruses and the evolution of host range and virulence. Infect. Genet. Evol. 21, 15–40 (2014).
Dobó, J., Swanson, R., Salvesen, G. S., Olson, S. T. & Gettins, P. G. Cytokine response modifier a inhibition of initiator caspases results in covalent complex formation and dissociation of the caspase tetramer. J. Biol. Chem. 281, 38781–38790 (2006).
Melo-Silva, C. R. et al. The ectromelia virus SPI-2 protein causes lethal mousepox by preventing NK cell responses. J. Virol. 85, 11170–11182 (2011).
Turner, P. C. et al. Myxoma virus Serp2 is a weak inhibitor of granzyme B and interleukin-1β-converting enzyme in vitro and unlike CrmA cannot block apoptosis in cowpox virus-infected cells. J. Virol. 73, 6394–6404 (1999).
Stennicke, H. R., Ryan, C. A. & Salvesen, G. S. Reprieval from execution: the molecular basis of caspase inhibition. Trends Biochem. Sci. 27, 94–101 (2002).
Ishimwe, E., Hodgson, J. J., Clem, R. J. & Passarelli, A. L. Reaching the melting point: degradative enzymes and protease inhibitors involved in baculovirus infection and dissemination. Virology 479–480, 637–649 (2015).
Gerlic, M. et al. Vaccinia virus F1L protein promotes virulence by inhibiting inflammasome activation. Proc. Natl Acad. Sci. USA 110, 7808–7813 (2013).
Gregory, S. M. et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331, 330–334 (2011).
Komune, N., Ichinohe, T., Ito, M. & Yanagi, Y. Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J. Virol. 85, 13019–13026 (2011).
Chen, I. Y. & Ichinohe, T. Response of host inflammasomes to viral infection. Trends Microbiol. 23, 55–63 (2015).
Marc, D. Influenza virus non-structural protein NS1: interferon antagonism and beyond. J. Gen. Virol. 95, 2594–2611 (2014).
Stasakova, J. et al. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1β and 18. J. Gen. Virol. 86, 185–195 (2005).
Xiang, Y. & Moss, B. IL-18 binding and inhibition of interferon-γ induction by human poxvirus-encoded proteins. Proc. Natl Acad. Sci. USA 96, 11537–11542 (1999).
Born, T. L. et al. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J. Immunol. 164, 3246–3254 (2000).
Esteban, D. J., Nuara, A. A. & Buller, R. M. Interleukin-18 and glycosaminoglycan binding by a protein encoded by variola virus. J. Gen. Virol. 85, 1291–1299 (2004).
Smith, V. P., Bryant, N. A. & Alcamí, A. Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J. Gen. Virol. 81, 1223–1230 (2000).
Xiang, Y. & Moss, B. Correspondence of the functional epitopes of poxvirus and human interleukin-18-binding proteins. J. Virol. 75, 9947–9954 (2001).
Nazarian, S. H. et al. Yaba monkey tumor virus encodes a functional inhibitor of interleukin-18. J. Virol. 82, 522–528 (2008).
Krumm, B., Meng, X., Wang, Z., Xiang, Y. & Deng, J. A unique bivalent binding and inhibition mechanism by the yatapoxvirus interleukin 18 binding protein. PLoS Pathog. 8, e1002876 (2012).
Spriggs, M. K. et al. Vaccinia and cowpox viruses encode a novel secreted interleukin-1-binding protein. Cell 71, 145–152 (1992).
Sivaraman, V., Pechous, R. D., Stasulli, N. M., Miao, E. A. & Goldman, W. E. Yersinia pestis activates both IL-1β and IL-1 receptor antagonist to modulate lung inflammation during pneumonic plague. PLoS Pathog. 11, e1004688 (2015).
Qu, Y. et al. Phosphorylation of NLRC4 is critical for inflammasome activation. Nature 490, 539–542 (2012).
Juliana, C. et al. Non-transcriptional priming and deubiquitination regulate NLRP3 inflammasome activation. J. Biol. Chem. 287, 36617–36622 (2012).
Yen, H., Sugimoto, N. & Tobe, T. Enteropathogenic Escherichia coli uses NleA to inhibit NLRP3 inflammasome activation. PLoS Pathog. 11, e1005121 (2015).
Fink, S. L. & Cookson, B. T. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell. Microbiol. 8, 1812–1825 (2006). This study shows that membrane pore formation mediates cellular lysis during pyroptosis.
Bergsbaken, T., Fink, S. L., den Hartigh, A. B., Loomis, W. P. & Cookson, B. T. Coordinated host responses during pyroptosis: caspase-1-dependent lysosome exocytosis and inflammatory cytokine maturation. J. Immunol. 187, 2748–2754 (2011). This study shows that lysosome exocytosis during pyroptosis releases antimicrobial molecules, and that the process of lysosome exocytosis is distinct from that of the release of pro-inflammatory cytokines.
Lotze, M. T. & Tracey, K. J. High-mobility group box1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat. Rev. Immunol. 5, 331–342 (2005).
Doz, E. et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MYD88 and IL-1R1/MYD88 signaling dependent. J. Immunol. 180, 1169–1178 (2008).
Amulic, B., Cazalet, C., Hayes, G. L., Metzler, K. D. & Zychlinsky, A. Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489 (2012).
Berends, E. T., Kuipers, A., Ravesloot, M. M., Urbanus, R. T. & Rooijakkers, S. H. Bacteria under stress by complement and coagulation. FEMS Microbiol. Rev. 38, 1146–1171 (2014).
Karmakar, M., Sun, Y., Hise, A. G., Rietsch, A. & Pearlman, E. Cutting edge: IL-1β processing during Pseudomonas aeruginosa infection is mediated by neutrophil serine proteases and is independent of NLRC4 and caspase-1. J. Immunol. 189, 4231–4235 (2012).
Franklin, B. S. et al. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat. Immunol. 15, 727–737 (2014).
Mosser, D. M. & Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Barker, J. R. et al. The Francisella tularensis pathogenicity island encodes a secretion system that is required for phagosome escape and virulence. Mol. Microbiol. 74, 1459–1470 (2009).
Burkinshaw, B. J. & Strynadka, N. C. Assembly and structure of the T3SS. Biochim. Biophys. Acta 1843, 1649–1663 (2014).
Sun, Y. H., Rolán, H. G. & Tsolis, R. M. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J. Biol. Chem. 282, 33897–33901 (2007). This study demonstrates the SPI-1 T3SS-dependent translocation of flagellin from Salmonella spp. into the cytosol of infected macrophages.
Molofsky, A. B. et al. Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J. Exp. Med. 203, 1093–1104 (2006).
Alvarez-Martinez, C. E. & Christie, P. J. Biological diversity of prokaryotic type IV secretion systems. Microbiol. Mol. Biol. Rev. 73, 775–808 (2009).
Byrne, B. G., Dubuisson, J. F., Joshi, A. D., Persson, J. J. & Swanson, M. S. Inflammasome components coordinate autophagy and pyroptosis as macrophage responses to infection. mBio 4, e00620-12 (2013).
Shi, C. S. et al. Activation of autophagy by inflammatory signals limits IL-1β production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 13, 255–263 (2012).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972). The first description of apoptosis as a pathway that leads to cell death, the Greek origins and correct pronunciation of apoptosis, the recognition of other forms of cell death or pathways that lead to cell death, the first description of the term 'necrosis' to describe dead cells and the end state that results from the execution of cell death pathways.
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nat. Rev. Microbiol. 7, 99–109 (2009).
Bergsbaken, T. & Cookson, B. T. Innate immune response during Yersinia infection: critical modulation of cell death mechanisms through phagocyte activation. J. Leukoc. Biol. 86, 1153–1158 (2009).
Monack, D. M., Mecsas, J., Ghori, N. & Falkow, S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc. Natl Acad. Sci. USA 94, 10385–10390 (1997).
Ruckdeschel, K. et al. Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect. Immun. 65, 4813–4821 (1997).
Zhang, Y. & Bliska, J. B. Role of Toll-like receptor signaling in the apoptotic response of macrophages to Yersinia infection. Infect. Immun. 71, 1513–1519 (2003).
Haase, R. et al. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J. Immunol. 171, 4294–4303 (2003).
Bergsbaken, T. & Cookson, B. T. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 3, e161 (2007). This study demonstrates that the activation state of macrophages is a crucial parameter that controls the use of cell death pathways in response to infection with Yersinia spp.
Levine, B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162 (2005).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Schnaith, A. et al. Staphylococcus aureus subvert autophagy for induction of caspase-independent host cell death. J. Biol. Chem. 282, 2695–2706 (2007).
Huang, J. & Brumell, J. H. Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12, 101–114 (2014).
Doitsh, G. et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514 (2014).
Stewart, M. K. & Cookson, B. T. Non-genetic diversity shapes infectious capacity and host resistance. Trends Microbiol. 20, 461–466 (2012).
Knodler, L. A. et al. Dissemination of invasive Salmonella via bacterial-induced extrusion of mucosal epithelia. Proc. Natl Acad. Sci. USA 107, 17733–17738 (2010).
Acknowledgements
The authors acknowledge funding from the US National Institutes of Health and apologize to those colleagues whose work could not be cited owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Pattern recognition receptors
-
(PRRs). Host proteins that recognize pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs). PRRs often specifically bind to pathogen-derived molecules, such as lipopolysaccharide.
- Pathogen-associated molecular patterns
-
(PAMPs). Pathogen-derived molecules that are recognized by pattern recognition receptors (PRRs).
- Damage-associated molecular patterns
-
(DAMPs). The mammalian immune system has evolved to recognize certain host-derived molecules as hallmarks of cellular damage when these molecules are detected outside of their usual cellular compartment.
- Caspase
-
A cysteine protease that cleaves after an aspartic acid residue and initiates and executes cell death programmes. All caspases are produced as catalytically inactive zymogens and generally require cleavage for activity. Pro-inflammatory caspases include caspase 1, caspase 4, caspase 5, caspase 11 and caspase 12, and are associated with pyroptosis and the maturation of the cytokines interleukin-1β (IL-1β) and IL-18.
- Caspase activation and recruitment domain
-
(CARD). Homotypic interactions between CARDs mediate the binding of caspase 1 to other CARD-containing proteins, such as apoptosis-associated speck-like protein containing a CARD (ASC).
- Type III injectisome secretion systems
-
(T3SSs). A syringe-like organelle through which molecules can be translocated from the interior of a bacterium to the host cell cytosol. The periplasmic portion of this channel is thought to be formed of the rod protein, whereas the extracellular portion of the syringe shaft is a homopolymer of the needle protein.
- Flagellin
-
Monomeric protein subunit of the bacterial flagellum.
- Pyrin domain
-
(PYD). Homotypic interactions between PYDs mediate the binding of apoptosis-associated speck-like protein containing a CARD (ASC) to other PYD-containing proteins, such as NBD-, LRR- and pyrin domain-containing 3 (NLRP3), absent in melanoma 2 (AIM2) and interferon-γ-inducible protein 16 (IFI16).
- Type I IFN responses
-
Type I interferons (IFNα and IFNβ) are produced and secreted in response to the recognition of pathogen-associated molecular patterns (PAMPs) by most Toll-like receptors (TLRs) and by numerous intracellular nucleic acid receptors. The binding of type I IFNs to their cognate receptor on infected and neighbouring cells activates the transcription of hundreds of interferon stimulated genes (ISGs), which inhibit viral replication and spread, induce apoptosis in cells that are infected, and modulate adaptive immunity.
- Serine protease inhibitors
-
(Serpins). Serpins share specific tertiary structural characteristics and inhibit serine proteases through a suicide substrate mechanism in which the serpin forms a covalent bond with the protease.
- Chick embryo chorioallantoic membrane
-
A vascular membrane found in the amniote egg that is used for the isolation, propagation and study of some viruses.
- Reassorted
-
The genome of influenza virus is composed of eight segments of negative-sense single-stranded RNA. Reassortment of the genome occurs when segments from different influenza strains are packaged together into a single virion.
Rights and permissions
About this article
Cite this article
Stewart, M., Cookson, B. Evasion and interference: intracellular pathogens modulate caspase-dependent inflammatory responses. Nat Rev Microbiol 14, 346–359 (2016). https://doi.org/10.1038/nrmicro.2016.50
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.50
This article is cited by
-
Pathogen-associated T follicular helper cell plasticity is critical in anti-viral immunity
Science China Life Sciences (2022)
-
NF-κB/ROS and ERK pathways regulate NLRP3 inflammasome activation in Listeria monocytogenes infected BV2 microglia cells
Journal of Microbiology (2021)
-
Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death
Nature Communications (2017)
-
Controlled detonation: evolution of necroptosis in pathogen defense
Immunology & Cell Biology (2017)
-
Programmed cell death as a defence against infection
Nature Reviews Immunology (2017)