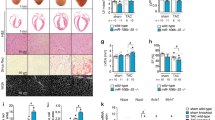
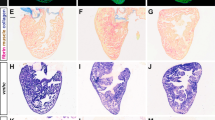
Key Points
-
In response to myocardial infarction, damaged adult cardiomyocytes are replaced by activated fibroblasts that form a fibrotic scar, leading to reduced cardiac function and heart failure. As the adult heart has limited regenerative capacity, there is a need to develop innovative strategies to enhance cardiac repair and regeneration.
-
Cellular replacement strategies for heart repair, in which stem cells and other cell types are injected directly into the injured heart or into the coronary circulation, have shown modest beneficial effects on cardiac function. An alternative approach is to reprogramme non-muscle cells in the injured heart to adopt a cardiac fate.
-
Positive cell cycle regulators are highly expressed in the embryonic heart and downregulated in the adult heart. Activation of various signalling pathways in the heart can modestly reactivate proliferation in adult cardiomyocytes.
-
Identification of transcription factors and microRNAs that control heart formation has enabled reprogramming of non-muscle cells into cardiomyocytes and other cell types of the heart. Following injury, in vivo reprogramming of non-myocytes into cardiomyocytes has improved heart function in mice.
-
Epicardial cells are activated following cardiac injury and have the potential to differentiate into various cell types, offering a niche that can be targeted with small molecules. This provides an attractive approach for regenerative medicine.
-
The inflammatory response has a role in cardiac repair following injury. Several studies have shed light on both positive and negative roles of the inflammatory response in tissue repair and regeneration.
Abstract
As the adult mammalian heart has limited potential for regeneration and repair, the loss of cardiomyocytes during injury and disease can result in heart failure and death. The cellular processes and regulatory mechanisms involved in heart growth and development can be exploited to repair the injured adult heart through 'reawakening' pathways that are active during embryogenesis. Heart function has been restored in rodents by reprogramming non-myocytes into cardiomyocytes, by expressing transcription factors (GATA4, HAND2, myocyte-specific enhancer factor 2C (MEF2C) and T-box 5 (TBX5)) and microRNAs (miR-1, miR-133, miR-208 and miR-499) that control cardiomyocyte identity. Stimulating cardiomyocyte dedifferentiation and proliferation by activating mitotic signalling pathways involved in embryonic heart growth represents a complementary approach for heart regeneration and repair. Recent advances in understanding the mechanistic basis of heart development offer exciting opportunities for effective therapies for heart failure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lopez, A. D., Mathers, C. D., Ezzati, M., Jamison, D. T. & Murray, C. J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet 367, 1747–1757 (2006).
Hill, J. A. & Olson, E. N. Cardiac plasticity. N. Engl. J. Med. 358, 1370–1380 (2008).
Segers, V. F. & Lee, R. T. Stem-cell therapy for cardiac disease. Nature 451, 937–942 (2008).
Laflamme, M. A. & Murry, C. E. Heart regeneration. Nature 473, 326–335 (2011).
Wollert, K. C. & Drexler, H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nature Rev. Cardiol. 7, 204–215 (2010).
Anversa, P., Kajstura, J., Rota, M. & Leri, A. Regenerating new heart with stem cells. J. Clin. Invest. 123, 62–70 (2013).
Passier, R., van Laake, L. W. & Mummery, C. L. Stem-cell-based therapy and lessons from the heart. Nature 453, 322–329 (2008).
Ptaszek, L. M., Mansour, M., Ruskin, J. N. & Chien, K. R. Towards regenerative therapy for cardiac disease. Lancet 379, 933–942 (2012).
Mercola, M., Ruiz-Lozano, P. & Schneider, M. D. Cardiac muscle regeneration: lessons from development. Genes Dev. 25, 299–309 (2011).
Buckingham, M., Meilhac, S. & Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nature Rev. Genet. 6, 826–835 (2005).
Olson, E. N. Gene regulatory networks in the evolution and development of the heart. Science 313, 1922–1927 (2006).
Srivastava, D. Making or breaking the heart: from lineage determination to morphogenesis. Cell 126, 1037–1048 (2006). References 10, 11 and 12 review the genetic circuits that drive the evolution and development of the heart.
Ieda, M. et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142, 375–386 (2010).
Qian, L. et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature 485, 593–598 (2012).
Song, K. et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 485, 599–604 (2012). References 13, 14 and 15 describe in vitro and in vivo reprogramming of non-muscle cells into cardiomyocytes.
Aguirre, A., Sancho-Martinez, I. & Izpisua Belmonte, J. C. Reprogramming toward heart regeneration: stem cells and beyond. Cell Stem Cell 12, 275–284 (2013).
Engel, F. B. et al. p38 MAP kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes Dev. 19, 1175–1187 (2005).
Kuhn, B. et al. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nature Med. 13, 962–969 (2007).
Bersell, K., Arab, S., Haring, B. & Kuhn, B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138, 257–270 (2009).
Heallen, T. et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458–461 (2011).
Xin, M. et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci. Signal. 4, ra70 (2011).
von Gise, A. et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl Acad. Sci. USA 109, 2394–2399 (2012). References 20, 21 and 22 show that the Hippo signalling pathway governs cardiomyocyte proliferation.
Porrello, E. R. et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ. Res. 109, 670–679 (2011).
Eulalio, A. et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature 492, 376–381 (2012). Identifies miRNAs that induce cardiomyocyte proliferation.
Garcia-Martinez, V. & Schoenwolf, G. C. Primitive-streak origin of the cardiovascular system in avian embryos. Dev. Biol. 159, 706–719 (1993).
Tam, P. P., Parameswaran, M., Kinder, S. J. & Weinberger, R. P. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development 124, 1631–1642 (1997).
Garry, D. J. & Olson, E. N. A common progenitor at the heart of development. Cell 127, 1101–1104 (2006).
Bruneau, B. G. Signaling and transcriptional networks in heart development and regeneration. Cold Spring Harb. Perspect. Biol. 5, a008292 (2013).
Ahuja, P., Sdek, P. & MacLellan, W. R. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol. Rev. 87, 521–544 (2007).
Li, F., Wang, X., Capasso, J. M. & Gerdes, A. M. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 28, 1737–1746 (1996).
Maillet, M., van Berlo, J. H. & Molkentin, J. D. Molecular basis of physiological heart growth: fundamental concepts and new players. Nature Rev. Mol. Cell Biol. 14, 38–48 (2013).
Bergmann, O. et al. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102 (2009).
Kajstura, J. et al. Cardiomyogenesis in the adult human heart. Circ. Res. 107, 305–315 (2010).
Senyo, S. E. et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436 (2013).
Poss, K. D., Wilson, L. G. & Keating, M. T. Heart regeneration in zebrafish. Science 298, 2188–2190 (2002). The first study to show that the adult zebrafish heart can regenerate.
Lepilina, A. et al. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619 (2006).
Jopling, C. et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609 (2010).
Kikuchi, K. et al. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 464, 601–605 (2010). References 37 and 38 demonstrate that zebrafish heart regeneration involves the proliferation of existing cardiomyocytes.
Fishman, M. C. & Olson, E. N. Parsing the heart: genetic modules for organ assembly. Cell 91, 153–156 (1997).
Wills, A. A., Holdway, J. E., Major, R. J. & Poss, K. D. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 135, 183–192 (2008).
Porrello, E. R. et al. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080 (2011). The first study to show that mammalian heart regeneration can occur transiently following birth.
Porrello, E. R. et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc. Natl Acad. Sci. USA 110, 187–192 (2013).
Pasumarthi, K. B. & Field, L. J. Cardiomyocyte cell cycle regulation. Circ. Res. 90, 1044–1054 (2002).
Chaudhry, H. W. et al. Cyclin A2 mediates cardiomyocyte mitosis in the postmitotic myocardium. J. Biol. Chem. 279, 35858–35866 (2004).
Pasumarthi, K. B., Nakajima, H., Nakajima, H. O., Soonpaa, M. H. & Field, L. J. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ. Res. 96, 110–118 (2005).
Katz, E. B. et al. Cardiomyocyte proliferation in mice expressing α-cardiac myosin heavy chain–SV40 T-antigen transgenes. Am. J. Physiol. 262, H1867–H1876 (1992).
Kubin, T. et al. Oncostatin M is a major mediator of cardiomyocyte dedifferentiation and remodeling. Cell Stem Cell 9, 420–432 (2011).
Engel, F. B., Hsieh, P. C., Lee, R. T. & Keating, M. T. FGF1/p38 MAP kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proc. Natl Acad. Sci. USA 103, 15546–15551 (2006).
Liang, Q. & Molkentin, J. D. Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models. J. Mol. Cell. Cardiol. 35, 1385–1394 (2003).
Cuevas, P. et al. Fibroblast growth factor-1 prevents myocardial apoptosis triggered by ischemia reperfusion injury. Eur. J. Med. Res. 2, 465–468 (1997).
Grego-Bessa, J. et al. Notch signaling is essential for ventricular chamber development. Dev. Cell 12, 415–429 (2007).
Liu, N. & Olson, E. N. MicroRNA regulatory networks in cardiovascular development. Dev. Cell 18, 510–525 (2010).
Small, E. M. & Olson, E. N. Pervasive roles of microRNAs in cardiovascular biology. Nature 469, 336–342 (2011).
Hullinger, T. G. et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ. Res. 110, 71–81 (2012).
Pan, D. The Hippo signaling pathway in development and cancer. Dev. Cell 19, 491–505 (2010).
Zhao, B., Tumaneng, K. & Guan, K. L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature Cell Biol. 13, 877–883 (2011).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). References 57 and 58 demonstrate that four transcription factors can induce iPS cells from fibroblasts.
Marchetto, M. C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Zhang, J. et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31–45 (2011).
Liu, G. H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson–Gilford progeria syndrome. Nature 472, 221–225 (2011).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Carvajal-Vergara, X. et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 465, 808–812 (2010).
Priori, S. G., Napolitano, C., Di Pasquale, E. & Condorelli, G. Induced pluripotent stem cell-derived cardiomyocytes in studies of inherited arrhythmias. J. Clin. Invest. 123, 84–91 (2013).
Nakamura, K., Hirano, K. & Wu, S. M. iPS cell modeling of cardiometabolic diseases. J. Cardiovasc. Transl. Res. 6, 46–53 (2013).
Bellin, M., Marchetto, M. C., Gage, F. H. & Mummery, C. L. Induced pluripotent stem cells: the new patient? Nature Rev. Mol. Cell Biol. 13, 713–726 (2012).
Yoshida, Y. & Yamanaka, S. iPS cells: a source of cardiac regeneration. J. Mol. Cell. Cardiol. 50, 327–332 (2011).
Efe, J. A. et al. Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nature Cell Biol. 13, 215–222 (2011).
Islas, J. F. et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl Acad. Sci. USA 109, 13016–13021 (2012).
Jugdutt, B. I. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation 108, 1395–1403 (2003).
Chen, J. X. et al. Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ. Res. 111, 50–55 (2012).
Addis, R. C. et al. Optimization of direct fibroblast reprogramming to cardiomyocytes using calcium activity as a functional measure of success. J. Mol. Cell. Cardiol. 60, 97–106 (2013).
Jayawardena, T. M. et al. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 110, 1465–1473 (2012).
Evans, S. M., Tai, L. J., Tan, V. P., Newton, C. B. & Chien, K. R. Heterokaryons of cardiac myocytes and fibroblasts reveal the lack of dominance of the cardiac muscle phenotype. Mol. Cell. Biol. 14, 4269–4279 (1994).
Takeuchi, J. K. & Bruneau, B. G. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459, 708–711 (2009).
Minasi, M. G. et al. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129, 2773–2783 (2002).
Acharya, A. et al. The bHLH transcription factor Tcf21 is required for lineage-specific EMT of cardiac fibroblast progenitors. Development 139, 2139–2149 (2012).
Nam, Y. J. et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc. Natl Acad. Sci. USA 110, 5588–5593 (2013).
Kapoor, N., Galang, G., Marban, E. & Cho, H. C. Transcriptional suppression of connexin43 by TBX18 undermines cell–cell electrical coupling in postnatal cardiomyocytes. J. Biol. Chem. 286, 14073–14079 (2011).
Rentschler, S. et al. Myocardial Notch signaling reprograms cardiomyocytes to a conduction-like phenotype. Circulation 126, 1058–1066 (2012).
Wang, Z., Wang, D. Z., Pipes, G. C. & Olson, E. N. Myocardin is a master regulator of smooth muscle gene expression. Proc. Natl Acad. Sci. USA 100, 7129–7134 (2003).
Chen, J., Kitchen, C. M., Streb, J. W. & Miano, J. M. Myocardin: a component of a molecular switch for smooth muscle differentiation. J. Mol. Cell. Cardiol. 34, 1345–1356 (2002).
Du, K. L. et al. Myocardin is a critical serum response factor cofactor in the transcriptional program regulating smooth muscle cell differentiation. Mol. Cell. Biol. 23, 2425–2437 (2003).
Li, S., Wang, D. Z., Wang, Z., Richardson, J. A. & Olson, E. N. The serum response factor coactivator myocardin is required for vascular smooth muscle development. Proc. Natl Acad. Sci. USA 100, 9366–9370 (2003).
Pipes, G. C., Creemers, E. E. & Olson, E. N. The myocardin family of transcriptional coactivators: versatile regulators of cell growth, migration, and myogenesis. Genes Dev. 20, 1545–1556 (2006).
Margariti, A. et al. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proc. Natl Acad. Sci. USA 109, 13793–13798 (2012).
Kurian, L. et al. Conversion of human fibroblasts to angioblast-like progenitor cells. Nature Methods 10, 77–83 (2013).
Ginsberg, M. et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFβ suppression. Cell 151, 559–575 (2012).
Sucov, H. M., Gu, Y., Thomas, S., Li, P. & Pashmforoush, M. Epicardial control of myocardial proliferation and morphogenesis. Pediatr. Cardiol. 30, 617–625 (2009).
Zhou, B. et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Invest. 121, 1894–1904 (2011).
Katz, T. C. et al. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 22, 639–650 (2012).
Majesky, M. W. Development of coronary vessels. Curr. Top. Dev. Biol. 62, 225–259 (2004).
Red-Horse, K., Ueno, H., Weissman, I. L. & Krasnow, M. A. Coronary arteries form by developmental reprogramming of venous cells. Nature 464, 549–553 (2010).
Huang, G. N. et al. C/EBP transcription factors mediate epicardial activation during heart development and injury. Science 338, 1599–1603 (2012).
Rinkevich, Y. et al. Identification and prospective isolation of a mesothelial precursor lineage giving rise to smooth muscle cells and fibroblasts for mammalian internal organs, and their vasculature. Nature Cell Biol. 14, 1251–1260 (2012).
Acharya, A., Baek, S. T., Banfi, S., Eskiocak, B. & Tallquist, M. D. Efficient inducible Cre-mediated recombination in Tcf21 cell lineages in the heart and kidney. Genesis 49, 870–877 (2011).
Kikuchi, K. et al. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 138, 2895–2902 (2011).
Russell, J. L., Goetsch, S. C., Aguilar, H. R., Frantz, D. E. & Schneider, J. W. Targeting native adult heart progenitors with cardiogenic small molecules. ACS Chem. Biol. 7, 1067–1076 (2012).
Heredia, J. E. et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell 153, 376–388 (2013).
Kharraz, Y., Guerra, J., Mann, C. J., Serrano, A. L. & Munoz-Canoves, P. Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators Inflamm. 2013, 491497 (2013).
Saclier, M., Cuvellier, S., Magnan, M., Mounier, R. & Chazaud, B. Monocyte/macrophage interactions with myogenic precursor cells during skeletal muscle regeneration. FEBS J. http://dx.doi.org/10.1111/febs.12166 (2013).
Frangogiannis, N. G. The immune system and cardiac repair. Pharmacol. Res. 58, 88–111 (2008).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Kyritsis, N. et al. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338, 1353–1356 (2012).
Lee, J. et al. Activation of innate immunity is required for efficient nuclear reprogramming. Cell 151, 547–558 (2012).
Kawamura, M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126, S29–S37 (2012).
Chen, H. S., Kim, C. & Mercola, M. Electrophysiological challenges of cell-based myocardial repair. Circulation 120, 2496–2508 (2009).
Zhang, Y. M., Hartzell, C., Narlow, M. & Dudley, S. C. Jr. Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation 106, 1294–1299 (2002).
Lee, R. J., Hinson, A., Helgerson, S., Bauernschmitt, R. & Sabbah, H. N. Polymer-based restoration of left ventricular mechanics. Cell Transplant. 22, 529–533 (2013).
Badylak, S. F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Zhang, R. et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. http://dx.doi.org/10.1038/nature12322 (2013).
Acknowledgements
The authors thank G. Huang, Y.-J. Nam, A. Aurora and K. Song for constructive scientific discussions. They thank J. Cabrera for assistance with figures. Work in the author's laboratory was supported by grants from the National Institutes of Health, the Robert A. Welch Foundation (grant I-0025), the Leducq Foundation-Transatlantic Network of Excellence in Cardiovascular Research Program, the American Heart Association-Jon Holden DeHaan Foundation and the Cancer Prevention and Research Institute of Texas (CPRIT). M.X. was supported by a Beginning Grant-in-Aid from the SouthWest Affiliate of the American Heart Association.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Paracrine effects
-
The effects of a signalling factor secreted by one cell on a nearby cell.
- Cardiogenesis
-
The development of the embryonic heart.
- Myocardial infarction
-
Interruption of the blood flow to the heart, causing cell death and heart damage. Also known as heart attack.
- Lineage tracing
-
A genetic tool used to trace all progeny originating from a single cell.
- Cre–loxP recombination system
-
Tissue-specific expression of Cre recombinase to carry out targeted gene deletion.
- Ventricle apex
-
Anatomically, the lowest portion of the heart.
- Myocardial ischaemia–reperfusion
-
Restoration of the blood supply to the heart tissue that is ischaemic due to a decrease in this.
- Trabecular cardiomyocytes
-
Highly organized cardiomyocytes that form 'projections' into the lumen of the heart ventricles to increase surface area. They facilitate contractility of the heart.
- Locked nucleic acid (LNA)-modified anti-miRNAs
-
Chemically modified, single-stranded RNA oligonucleotides that contain an extra bridge connecting the 2′ oxygen and 4′ carbon, which 'locks' the ribose in the 3′-endo conformation. This results in high stability and affinity to inactivate specific miRNAs.
- Subcompact ventricular myocardial layers
-
The thick muscular walls of the heart ventricles.
- Teratogenicity
-
The capability of producing congenital anomalies.
- Heterokaryons
-
A cell that contains multiple genetically different nuclei.
- Gap junctions
-
A specialized intercellular connection that directly connects the cytoplasm of two cells, allowing various molecules and ions to pass freely between cells.
- Venous plexus
-
A congregation of multiple veins.
Rights and permissions
About this article
Cite this article
Xin, M., Olson, E. & Bassel-Duby, R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol 14, 529–541 (2013). https://doi.org/10.1038/nrm3619
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3619
This article is cited by
-
Proteomic analysis of neonatal mouse hearts shows PKA functions as a cardiomyocyte replication regulator
Proteome Science (2023)
-
RNA modification in cardiovascular disease: implications for therapeutic interventions
Signal Transduction and Targeted Therapy (2023)
-
Human organoids in basic research and clinical applications
Signal Transduction and Targeted Therapy (2022)
-
Mesp1 controls the chromatin and enhancer landscapes essential for spatiotemporal patterning of early cardiovascular progenitors
Nature Cell Biology (2022)
-
Heart-on-Chip for Combined Cellular Dynamics Measurements and Computational Modeling Towards Clinical Applications
Annals of Biomedical Engineering (2022)