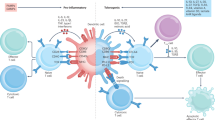
Abstract
Evidence from animal models has shown that the transfer of regulatory T cells or tolerogenic dendritic cells can prevent or cure transplant rejection or re-establish self-tolerance in autoimmune disease. The potential of these tolerogenic cells for the treatment of T-cell-mediated diseases in humans has gained momentum in recent years. Here, Nature Reviews Immunology asks four leading researchers to provide their view on the future of cell-based tolerogenic therapy and to highlight some important issues that must be considered before this form of immunotherapy can become a reality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
FURTHER READING
Chatenoud, L. & Bluestone, J. A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nature Rev. Immunol. 7, 622–632 (2007)
Roncarolo, M.-G. & Battaglia, M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nature Rev. Immunol. 7, 585–598 (2007)
Morelli, A. E. & Thomson, A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nature Rev. Immunol. 7, 610–621 (2007)
Acknowledgements
The authors thank colleagues and laboratory members who have persevered in this exciting field. A.W.T.'s work is supported by grants from the National Institutes of Health, USA.
Ethics declarations
Competing interests
J.A.B. has both research and consulting agreements with BD Biosciences related to human regulatory cell studies.
Related links
Glossary
- Combination immunotherapy
-
The use of more than one immunomodulatory treatment in combination for the treatment of autoimmune diseases or transplantation. The treatments would target different aspects of the immune response and immune function, for example, the induction of regulatory T cells combined with killing of effector or memory T cells.
- 'Designer' regulatory T cell
-
Regulatory T cells that have been modified by gene transfer to selectively express preferred proteins including, for example, antigen-specific T-cell receptors, homing receptors, cytokines and suicide genes.
- Glucocorticoids
-
A group of compounds that belongs to the corticosteroid family. These compounds can either be naturally produced (hormones) or synthetic. They affect metabolism and have anti-inflammatory and immunosuppressive effects. Many synthetic glucocorticoids (for example, dexamethasone) are used in clinical medicine as anti-inflammatory drugs.
- Good manufacturing practice
-
(GMP). A practice that ensures that products are consistently manufactured and controlled to the quality standards that are appropriate to their intended use.
- Graft-versus-host disease
-
(GVHD). Tissue damage in a recipient of allogeneic tissue (usually a bone-marrow transplant) that results from the activity of donor cytotoxic T lymphocytes recognizing the tissues of the recipient as foreign. GVHD varies markedly in extent, but it can be life threatening in severe cases. Damage to the liver, skin and gut mucosa are common clinical manifestations.
- Humanized mice
-
Immunodeficient mice that are engrafted with human haematopoietic cells or tissues, or mice that transgenically express human genes.
- Rapamycin
-
A prodrug that, when complexed with its intracellular receptor FK506-binding protein 12, inhibits downstream signalling from mTOR (mammalian target of rapamycin) proteins and impairs the maturation and T-cell allostimulatory function of dendritic cells and T-cell proliferation in response to interleukin-2.
- Suicide gene transduction
-
An innovative type of gene therapy that makes regulatory T cells commit suicide if they are mediating an unwanted activity. Some of the genes involved in this type of therapy include the gene for herpes simplex virus thymidine kinase, for the prodrug-activating enzyme carboxypeptidase G2 or for diptheria toxin.
- Type 1 diabetes
-
A chronic autoimmune disease that is characterized by the T-cell-mediated destruction of β-cells (which secrete insulin) in the pancreas. Individuals with type 1 diabetes develop hyperglycaemia and can develop diabetes-associated complications in multiple organ systems owing to lack of insulin. Insulitis in non-obese diabetic (NOD) mice is a model of type 1 diabetes.
Rights and permissions
About this article
Cite this article
Bluestone, J., Thomson, A., Shevach, E. et al. What does the future hold for cell-based tolerogenic therapy?. Nat Rev Immunol 7, 650–654 (2007). https://doi.org/10.1038/nri2137
Issue Date:
DOI: https://doi.org/10.1038/nri2137
This article is cited by
-
Adoptive Cell Therapy with Tregs to Improve Transplant Outcomes: the Promise and the Stumbling Blocks
Current Transplantation Reports (2016)
-
Challenges and approaches for the development of safer immunomodulatory biologics
Nature Reviews Drug Discovery (2013)
-
Comparative study of clinical grade human tolerogenic dendritic cells
Journal of Translational Medicine (2011)
-
Immunobiology of Transplantation: Impact on Targets for Large and Small Molecules
Clinical Pharmacology & Therapeutics (2011)
-
TREG cell control of autoimmune inflammation: a matter of timing?
Nature Reviews Rheumatology (2010)