Abstract
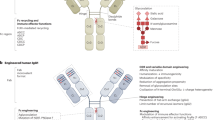
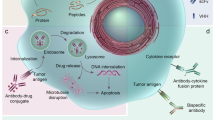
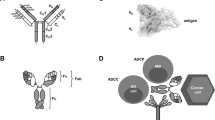
The potential of monoclonal antibodies to effectively treat cancer is beginning to be widely acknowledged. Advances in antibody engineering make it possible to produce various recombinant proteins that exploit the specificity of the antibody-combining site to manipulate tumour-related signalling, and to stimulate anti-tumour immune responses. Future advances in the field will rely on the improved identification of functional antibody targets to perturb cancer-relevant signalling, and by the improved selection of tumours that can be effectively treated. These advances will be complemented by the use of antibodies that induce clinically meaningful host-protective immune responses. But, can we afford this progress?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Ehrlich, P. Nobel Lectures, Physiology or Medicine 1901–1921. 304–320 (Elsevier Publishing Company, Amsterdam, 1967).
Witzig T. E. et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 20, 2453–2463 (2002).
Kaminski, M. S. et al. Radioimmunotherapy with iodine (131)I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 96, 1259–1266 (2000).
Sievers, E. L. et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93, 3678–3684 (1999).
Kreitman, R. J. et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 345, 241–247 (2001).
Kohler, G & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001).
Miller, R. A., Maloney, D. G., Warnke, R. & Levy, R. Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N. Engl. J. Med. 306, 517–522 (1982).
McLaughlin, P. et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 16, 2825–2833 (1998).
Osterborg, A. et al. Phase II multicenter study of human CD52 antibody in previously treated chronic lymphocytic leukemia. European Study Group of CAMPATH-1H Treatment in Chronic Lymphocytic Leukemia. J. Clin. Oncol. 15, 1567–1574 (1997).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Gibson, T. B., Ranganathan, A. & Grothey, A. Randomized phase III trial results of panitumumab, a fully human anti-epidermal growth factor receptor monoclonal antibody, in metastatic colorectal cancer. Clin. Colorectal Cancer 6, 13 (2006).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Hodi, F. S. et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA 100, 4712–4717 (2003).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
LoBuglio, A. F. et al. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc. Natl Acad. Sci. USA 86, 4220–4224 (1989).
Boulianne, G. L., Hozumi, N. & Shulman, M. J. Production of functional chimaeric mouse/human antibody. Nature 312, 634–646 (1984).
Morrison, S. L. et al. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc. Natl Acad. Sci. USA 81, 6851–6855 (1984).
Riechmann, L. et al. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
McCafferty, J. et al. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Weiner, L. M. Fully human therapeutic monoclonal antibodies. J. Immunother. 29, 1–9 (2006).
Gelderman, K. A., Tomlinson, S., Ross, G. D. & Gorter, A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 25, 158–164 (2004).
Shields, R. L. et al. High resolution mapping of the binding site on human IgG1 for Fc γ RI, Fc γ RII, Fc γ RIII, and FcRn and design of IgG1 variants with improved binding to the Fc γ R. J. Biol. Chem. 276, 6591–6604 (2001).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006).
Umana, P. et al. Engineered glycoforms of an antineuroblastoma IgG1 with optimized antibody-dependent cellular cytotoxic activity. Nature Biotechnol. 17, 176–180 (1999).
Mendelsohn, J. & Baselga, J. The EGF receptor family as targets for cancer therapy. Oncogene 19, 6550–6565 (2000).
Olayioye, M. A., Neve, R. M., Lane, H. A. & Hynes, N. E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19, 3159–3167 (2000).
Ferrara, N. & Kerbel, R. S. Angiogenesis as a therapeutic target. Nature 438, 967–974 (2005).
Holbro, T. Civenni, G. & Hynes, N. E. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 284, 99–110 (2003).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Yarden, Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37 Suppl. 4, S3–S8 (2001).
Tai, Y. T. et al. Human anti-CD40 antagonist antibody triggers significant antitumor activity against human multiple myeloma. Cancer Res. 65, 5898–5906 (2005).
Vonderheide, R. H. et al. Clinical activity and immune modulation in cancer patients treated with CP-870, 893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 25, 876–883 (2007).
Steplewski, Z., Lubeck M. D. & Koprowski, H. Human macrophages armed with murine immunoglobulin G2a antibodies to tumors destroy human cancer cells. Science 221, 865–867 (1983).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors: old friends and new family members. Immunity 24, 19–28 (2006).
Iannello, A. & Ahmad, A. Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 24, 487–499 (2005).
Clynes, R. A., Towers, T. L., Presta, L.G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 6, 443–446 (2000).
Weng, W. K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Gorter, A. & Meri, S. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol. Today 20, 576–582 (1999).
Trikha, M., Yan, L. & Nakada, M. T. Monoclonal antibodies as therapeutics in oncology. Curr. Opin. Biotechnol. 13, 609–614 (2002).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nature Biotechnol. 23, 1147–1157 (2005).
Whitehurst, A. W. et al. Synthetic lethal screen identification of chemosensitizer loci in cancer cells. Nature 446, 815–819 (2007).
Imai, K. & Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nature Rev. Cancer 6, 714–727 (2006).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and response to trastuzumab-based treatment in patients with HER-2/neu-positive metastatic breast cancer. Proc. AACR abstr. 4188, (2007).
McCall, A. M. et al. Increasing the affinity for tumor antigen enhances bispecific antibody cytotoxicity. J. Immunol. 166, 6112–6117 (2001).
Shahied, L. S. et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen-binding format. J. Biol. Chem. 279, 53907–53914 (2004).
Rafiq, K., Bergtold, A. & Clynes, R. Immune complex-mediated antigen presentation induces tumor immunity. J. Clin. Invest. 110, 71–79 (2002).
Korman, A. J., Peggs, K. S. & Allison, J. P. Checkpoint blockade in cancer immunotherapy. Adv. Immunol. 90, 297–339 (2006).
Sanderson, K. et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J. Clin. Oncol. 23, 741–750 (2005).
Sheridan, C. TeGenero fiasco prompts regulatory rethink. Nature Biotechnol. 24, 475–476 (2006).
Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005).
Blank, C. et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int. J. Cancer 119, 317–327 (2006).
Chung, K. Y. et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J. Clin. Oncol. 23, 1803–1810 (2005).
Adams, G. P. et al. High affinity restricts the localization and tumor penetration of single-chain Fv antibody molecules. Cancer Res. 61, 4750–4755 (2001).
Segal, D. M., Weiner, G. J. & Weiner, L. M. Bispecific antibodies in cancer therapy. Curr. Opin. Immunol. 11, 558–562 (1999).
Valone, F. H. et al. Phase Ia/Ib trial of bispecific antibody MDX-210 in patients with advanced breast or ovarian cancer that overexpresses the proto-oncogene HER-2/neu. J. Clin. Oncol. 13, 2281–2292 (1995).
Weiner, L. M. et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and FcγRIII. Cancer Res. 55, 4586–4593 (1995).
Amoroso, A. R. et al. Binding characteristics and antitumor properties of 1A10 bispecific antibody recognizing gp40 and human transferrin receptor. Cancer Res. 56, 113–120 (1996).
Lu, D. et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J. Biol. Chem. 280, 19665–19672 (2005).
Jackson, J. G. et al. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 64, 2601–2609 (2004).
Yokota, T., Milenic, D. E., Whitlow, M. & Schlom, J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 52, 3402–3408 (1992).
Wong, J. Y. et al. Pilot trial evaluating an 123I labeled 80-kilodalton engineered anticarcinoembryonic antigen antibody fragment (cT84.66 minibody) in patients with colorectal cancer. Clin. Cancer Res. 10, 5014–5021 (2004).
Adams, G. P. et al. Prolonged in vivo tumor retention of a human diabody targeting the extracellular domain of human HER2/neu. Brit. J. Cancer 77, 1405–1412 (1998).
Huston, J. S. et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl Acad. Sci. USA 85, 5879–5883 (1988).
Winkelmayer, W. C. et al. Health economic evaluations: the special case of end-stage renal disease treatment. Med. Decis. Making 22, 417–430 (2002).
Draviam, V. M. et al. A functional genomic screen identifies a role for TAO1 kinase in spindle-checkpoint signalling. Nature Cell Biol. 9, 556–564 (2007).
Janeway, C. A. Jr & Travers, P. (eds). Immunobiology – the immune system in health and disease. Current Biology Ltd, London, 1997.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Glossary
- Antibody valence
-
The number of different molecules an antibody can combine with at one time.
- B-cell idiotype
-
A unique immunoglobulin sequence variant, typically in the antibody complementarity-determining regions (CDRs).
- Bispecific antibodies
-
Antibodies with two distinct binding specificities (such as ERBB2 and CD16), which can be prepared by fusing two distinct hybridomas, through chemical conjugation, or using recombinant antibody engineering techniques.
- Complement
-
A heat-labile component of normal plasma that contains many proteins acting together to augment opsonization of targets by antibodies and promote antibody-mediated target destruction by forming membrane-attack complexes that form pores in target cell membranes, and also leads to clearance of immune complexes.
- Diabodies
-
Recombinant antibodies composed of two single-chain Fv molecules joined by a very short amino acid linker that 'forces' the protein into a divalent binding format that confers intermediate properties compared with the scFv and minibody formats.
- Minibodies
-
Recombinant antibodies composed of scFvs fused to antibody Fc domain elements to create a divalent molecule with pharmacological properties that more closely resemble those of intact IgG immunoglobulins.
- Single-chain Fv fragments
-
(scFv) Small (25 kDa) proteins that consist of variable-heavy and variable-light chain genes joined by a short amino acid linker that recapitulate an intact antibody binding site, but exhibit rapid tumour penetration and systemic clearance.
Rights and permissions
About this article
Cite this article
Weiner, L. Building better magic bullets — improving unconjugated monoclonal antibody therapy for cancer. Nat Rev Cancer 7, 701–706 (2007). https://doi.org/10.1038/nrc2209
Issue Date:
DOI: https://doi.org/10.1038/nrc2209
This article is cited by
-
In Silico Engineering Towards Enhancement of Bap–VHH Monoclonal Antibody Binding Affinity
International Journal of Peptide Research and Therapeutics (2019)
-
Antibody-dependent fragmentation is a newly identified mechanism of cell killing in vivo
Scientific Reports (2017)
-
Side-by-side analysis of five clinically tested anti-EpCAM monoclonal antibodies
Cancer Cell International (2010)
-
Treatment options for patients with triple-negative breast cancer
Journal of Hematology & Oncology (2010)