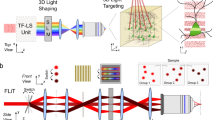
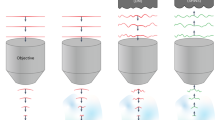
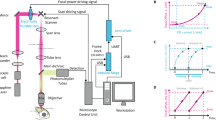
Abstract
Neuronal networks process information in a distributed, spatially heterogeneous manner that transcends the layout of electrodes. In contrast, directed and steerable light offers the potential to engage specific cells on demand. We present a unified framework for adapting microscopes to use light for simultaneous in vivo stimulation and recording of cells at fine spatiotemporal resolutions. We use straightforward optics to lock onto networks in vivo, to steer light to activate circuit elements and to simultaneously record from other cells. We then actualize this 'free' augmentation on both an 'open' two-photon microscope and a leading commercial one. By following this protocol, setup of the system takes a few days, and the result is a noninvasive interface to brain dynamics based on directed light, at a network resolution that was not previously possible and which will further improve with the rapid advance in development of optical reporters and effectors. This protocol is for physiologists who are competent with computers and wish to extend hardware and software to interface more fluidly with neuronal networks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Schwann, T. & Schleyden, M. Microscopical Researches into the Accordance in the Structure and Growth of Animals and Plants. (Sydenham Society, London, 1847).
O'Connor, D.H., Huber, D. & Svoboda, K. Reverse-engineering the mouse brain. Nature 461, 923–929 (2009).
Regehr, W.G., Connor, J.A. & Tank, D.W. Optical imaging of calcium accumulation in hippocampal pyramidal cells during synaptic activation. Nature 341, 533–536 (1989).
Svoboda, K., Denk, W., Kleinfeld, D. & Tank, D.W. In vivo dendritic calcium dynamics in neocortical pyramidal neurons. Nature 385, 161–165 (1997).
Smetters, D., Majewska, A. & Yuste, R. Detecting action potentials in neuronal populations with calcium imaging. Methods 18, 215–221 (1999).
Stosiek, C., Garaschuk, O., Holthoff, K. & Konnerth, A. In vivo two-photon calcium imaging of neuronal networks. Proc. Natl. Acad. Sci. USA 100, 7319–7324 (2003).
Shoham, S., O'Connor, D.H., Sarkisov, D.V. & Wang, S.S. Rapid neurotransmitter uncaging in spatially defined patterns. Nat. Methods 2, 837–843 (2005).
Pettit, D.L., Wang, S.S., Gee, K.R. & Augustine, G.J. Chemical two-photon uncaging: a novel approach to mapping glutamate receptors. Neuron 19, 465–471 (1997).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Cardin, J.A. et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459, 663–667 (2009).
Peron, S. & Svoboda, K. From cudgel to scalpel: toward precise neural control with optogenetics. Nat. Methods 8, 30–34 (2011).
Schummers, J., Yu, H. & Sur, M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320, 1638–1643 (2008).
Anastassiou, C.A., Perin, R., Markram, H. & Koch, C. Ephaptic coupling of cortical neurons. Nat. Neurosci. 14, 217–223 (2011).
Vervaeke, K., Lorincz, A., Nusser, Z. & Silver, R.A. Gap junctions compensate for sublinear dendritic integration in an inhibitory network. Science 335, 1624–1628 (2012).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Nikolenko, V., Poskanzer, K.E. & Yuste, R. Two-photon photostimulation and imaging of neural circuits. Nat. Methods 4, 943–950 (2007).
Guo, Z.V., Hart, A.C. & Ramanathan, S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 6, 891–896 (2009).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Valmianski, I. et al. Automatic identification of fluorescently labeled brain cells for rapid functional imaging. J. Neurophysiol. 104, 1803–1811 (2010).
Lillis, K.P., Eng, A., White, J.A. & Mertz, J. Two-photon imaging of spatially extended neuronal network dynamics with high temporal resolution. J. Neurosci. Methods 172, 178–184 (2008).
Grewe, B.F., Langer, D., Kasper, H., Kampa, B.M. & Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nat. Methods 7, 399–405 (2010).
Rothschild, G., Nelken, I. & Mizrahi, A. Functional organization and population dynamics in the mouse primary auditory cortex. Nat. Neurosci. 13, 353–360 (2010).
Wilson, N.R., Runyan, C.A., Wang, F.L. & Sur, M. Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348 (2012).
Gerits, A. et al. Optogenetically induced behavioral and functional network changes in primates. Curr. Biol. 22, 1722–1726 (2012).
Adamantidis, A.R. et al. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 31, 10829–10835 (2011).
Grinvald, A. & Hildesheim, R. VSDI: a new era in functional imaging of cortical dynamics. Nat. Rev. Neurosci. 5, 874–885 (2004).
Tian, L. et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 6, 875–881 (2009).
Chow, B.Y. et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 463, 98–102 (2010).
Li, Y. & Tsien, R.W. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat. Neurosci. 15, 1047–1053 (2012).
Zariwala, H.A. et al. A Cre-dependent GCaMP3 reporter mouse for neuronal imaging in vivo. J. Neurosci. 32, 3131–3141 (2012).
Madisen, L. et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat. Neurosci. 15, 793–802 (2012).
Sanjana, N.E. et al. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192 (2012).
Miyamichi, K. et al. Cortical representations of olfactory input by trans-synaptic tracing. Nature 472, 191–196 (2011).
Bock, D.D. et al. Network anatomy and in vivo physiology of visual cortical neurons. Nature 471, 177–182 (2011).
Alivisatos, A.P. et al. The brain activity map project and the challenge of functional connectomics. Neuron 74, 970–974 (2012).
Pologruto, T.A., Sabatini, B.L. & Svoboda, K. ScanImage: flexible software for operating laser scanning microscopes. Biomed. Eng. Online 2, 13 (2003).
Wagenaar, D.A. & Potter, S.M. A versatile all-channel stimulator for electrode arrays, with real-time control. J. Neural. Eng. 1, 39–45 (2004).
Asaad, W.F. & Eskandar, E.N. A flexible software tool for temporally-precise behavioral control in Matlab. J. Neurosci. Methods 174, 245–258 (2008).
Suter, B.A. et al. Ephus: multipurpose data acquisition software for neuroscience experiments. Front. Neural. Circuits 4, 100 (2010).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nat. Methods 9, 201–208 (2012).
Reddy, G.D. & Saggau, P. Fast three-dimensional laser scanning scheme using acousto-optic deflectors. J. Biomed. Opt. 10, 064038 (2005).
Kerlin, A.M., Andermann, M.L., Berezovskii, V.K. & Reid, R.C. Broadly tuned response properties of diverse inhibitory neuron subtypes in mouse visual cortex. Neuron 67, 858–871 (2010).
Oertner, T.G. The Lego-logic of optogenetics. Nat. Methods 8, 1011–1013 (2011).
Knopfel, T. Genetically encoded optical indicators for the analysis of neuronal circuits. Nat. Rev. Neurosci. 13, 687–700 (2012).
Yaksi, E. & Friedrich, R.W. Reconstruction of firing rate changes across neuronal populations by temporally deconvolved Ca2+ imaging. Nat. Methods 3, 377–383 (2006).
Vogelstein, J.T. et al. Spike inference from calcium imaging using sequential Monte Carlo methods. Biophys. J. 97, 636–655 (2009).
Petreanu, L., Huber, D., Sobczyk, A. & Svoboda, K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat. Neurosci. 10, 663–668 (2007).
Rickgauer, J.P. & Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Hirase, H., Nikolenko, V., Goldberg, J.H. & Yuste, R. Multiphoton stimulation of neurons. J. Neurobiol. 51, 237–247 (2002).
Hirase, H., Nikolenko, V. & Yuste, R. Multiphoton stimulation of neurons and spines. Cold Spring Harb. Protoc. 2012, 472–475 (2012).
Vaziri, A. & Emiliani, V. Reshaping the optical dimension in optogenetics. Curr. Opin. Neurobiol. 22, 128–137 (2012).
Acknowledgements
This work was supported by postdoctoral fellowships from the US National Institutes of Health (NIH) and the Simons Foundation (N.R.W.), an NIH predoctoral fellowship (C.A.R.) and grants from the NIH and the Simons Foundation (M.S.). We sincerely thank the ScanImage team at Janelia Farm, particularly V. Iyer, for providing an open platform on which others can build on and learn from. We thank K. Wang for assistance with the cell detection algorithm. We thank M. Goard for help with mirror feedback computation and S. El-Boustani for help with deconvolution. We thank V. Nikolenko for technical advice and Prairie Technologies, especially J. Rafter, E. Heins, P. Gustafson, M. Nazir and C. McCallum for working closely with us during the development of all of these techniques and providing a stable commercial platform with numerous points of entry for customization. Finally, we thank B. Land as a model in scientific communication, whose public sharing of documentation helped in our early days to learn key principles of computer control that could then be extended here.
Author information
Authors and Affiliations
Contributions
N.R.W. and J.S. contributed equally to the work. N.R.W. built the systems, wrote the software, performed experiments and analyses, and wrote the manuscript. J.S. conceived the ideas and chain of algorithms that would avail this system, performed analyses, helped develop the software and helped write the manuscript. C.A.R. improved the system and contributed critical in vivo experiments to validate it; S.X.Y. developed and calibrated the all-optical methodology; R.E.C. helped program critical algorithms for cell detection and image motion correction; Y.D. helped initiate and develop the all-optical methodology and M.S. encouraged these developments, steered their application, helped write the manuscript, and supported the refinement of a robust and general protocol applicable to other laboratories.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Platform for high-speed, targeted neuronal activation during concurrent two-photon imaging, electrophysiology and sensory stimulation (PDF 718 kb)
Supplementary Figure 2
Further calibration of targeted cell stimulation for optical activation in vivo (PDF 6102 kb)
Supplementary Figure 3
Specificity of activation of neurons in the z-plane (PDF 2556 kb)
Supplementary Data 1
Mirror positions on some microscopes will lag command signals by a significant, variable amount that is repeatable and predictable (PDF 414 kb)
Supplementary Data 2
Landing on the cells with the help of mirror feedback (PDF 424 kb)
Supplementary Data 3
By knowing the actual position of the mirrors at the point when fluorescence is detected, it is possible to attribute the intensity of the signal back to the cell bodies that emitted them (PDF 602 kb)
Supplementary Data 4
With careful calibration and proper attribution of fluorescence to spatial locations, delivering sensory stimuli across repeated trials in vivo will result in calcium traces collected from a given cell with a consistent selectivity for those sensory stimuli (PDF 2128 kb)
Supplementary Data 5
Analysis of measured fluorescence signals and signal-to-noise ratios. (PDF 650 kb)
Supplementary Data 6
Proper calibration of the system will also allow for clean calcium transients that can be deconvolved to inferred spike trains automatically using the software (PDF 1182 kb)
Supplementary Note 1
Overview of the Software (PDF 429 kb)
Supplementary Note 2
Controlling a Prairie Microscope with ScanImage Software (PDF 233 kb)
Rights and permissions
About this article
Cite this article
Wilson, N., Schummers, J., Runyan, C. et al. Two-way communication with neural networks in vivo using focused light. Nat Protoc 8, 1184–1203 (2013). https://doi.org/10.1038/nprot.2013.063
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.063
This article is cited by
-
Simultaneous high-speed imaging and optogenetic inhibition in the intact mouse brain
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.