Abstract
Exposure to methamphetamine (meth) can produce lasting memory impairments in humans and rodents. We recently demonstrated that extended access meth self-administration results in novel object recognition (NOR) memory deficits in rats. Recognition of novelty depends upon intact perirhinal (pRh) cortex function, which is compromised by meth-induced downregulation of GluN2B-containing N-methyl-D-aspartate (NMDA) receptors. NMDA receptors containing this subunit have a critical role in pRh long-term depression (LTD), one of the primary physiological processes thought to underlie object recognition memory. We hypothesized that meth-induced downregulation of GluN2B receptors would compromise pRh LTD, leading to loss of NOR memory. We found that meth self-administration resulted in an inability to induce pRh LTD following 1 Hz stimulation, an effect that was reversed with bath application of the NMDA receptor partial agonist D-cycloserine (DCS). In addition, pRh microinfusion of DCS restored meth-induced memory deficits. Furthermore, blockade of GluN2B-containing NMDA receptors with Ro 25-6981 prevented DCS restoration of pRh LTD in meth subjects. Thus, targeting pRh LTD may be a promising strategy to treat meth-induced cognitive impairment.
Similar content being viewed by others
INTRODUCTION
Despite profound negative consequences, methamphetamine (meth) abuse remains highly prevalent. In humans, chronic meth addiction often includes symptoms of impaired cognitive function in areas of episodic, verbal, and working memory (Scott et al, 2007; McCann et al, 2008; Salo et al, 2009). Several reviews have summarized the effects of repeated meth abuse on cognitive dysfunction and the clinical relevance of memory deficits (Scott et al, 2007; Hart et al, 2012; Dean et al, 2013). In general, meth causes cognitive decline in humans, particularly during early-to-middle adulthood (Dean et al, 2013). Importantly, the high incidence of cognitive dysfunction that occurs in chronic pathological meth addiction is distinct from acute or low-dose effects of meth (Wood et al, 2014). Also, memory impairment has been positively correlated with relapse probability, suggesting that abstinent meth addicts may reinitiate drug use as a means of alleviating cognitive impairment (Simon et al, 2004). Consequently, the mechanisms underlying meth-induced memory dysfunction are an important consideration for developing therapies for meth addiction.
In rodents, both non-contingent and operant meth self-administration (SA) paradigms impair recognition memory (Marshall and O'Dell, 2012). Here we use an extended access meth SA paradigm starting with short access (1 h) sessions and transition into chronic exposure with long access (6 h) sessions. Following meth SA, subjects then go through 7 days of forced abstinence. This paradigm reliably produces significant deficits in novel object and object-in-place recognition tasks not seen with short access (Rogers et al, 2008; Reichel and See, 2010; Reichel et al, 2011; Reichel et al, 2012) that have been postulated to represent key elements of episodic memory (Ennaceur, 2010).
Lesion studies in rodents have demonstrated that the perirhinal (pRh) cortex has an important role in temporal order and spatial memory processes, as do the hippocampus and medial prefrontal cortex (Barker et al, 2007; Barker and Warburton, 2011). However, pRh lesions disrupt the neural systems that regulate the discrimination of novel and familiar objects (Kealy and Commins, 2011). Moreover, studies have shown that the extent of pRh damage in lesion studies is positively correlated with the degree of object recognition impairment (Albasser et al, 2009). An emerging theory suggests that pRh neuron activity increases during novelty recognition and decreases as familiarity develops, eventually leading to the induction of pRh long-term depression (LTD; Warburton et al, 2013)
Behavioral pharmacology studies highlight the importance of pRh glutamatergic transmission in recognition memory as direct intracranial microinfusion of the N-methyl-D-aspartate (NMDA) receptor antagonist, (2R)-amino-5-phosphonovaleric acid, into the pRh just prior to task acquisition inhibits object recognition memory in rodents (Barker and Warburton, 2008; Warburton et al, 2013). Neurophysiological recording of the pRh cortex indicates that blockade of ionotropic glutamate receptors may inhibit object recognition memory by preventing the induction of NMDA receptor-dependent LTD (Cho et al, 2000; Griffiths et al, 2008). Further, neuroimaging of the pRh cortex in Macaques shows that blockade of glutamatergic transmission impairs recognition memory (Malkova et al, 2015).
We have previously shown that extended access meth SA followed by 7 days of withdrawal selectively downregulates the expression of the NMDA receptor subunit GluN2B, yet has no effect on expression of the GluN2A subunit, using a whole-cell lysate preparation of the pRh (Reichel et al, 2014). GluN2B-containing NMDA receptors mediate the induction of pRh LTD (Massey et al, 2004), one of the primary physiological processes believed to underlie recognition memory (Griffiths et al, 2008; Massey et al, 2008). Here we show that meth SA impaired recognition memory, blocked the induction of pRh LTD, and decreased GluN2B surface expression. Further, pharmacological activation of NMDA receptors with the partial agonist D-cycloserine (DCS) reversed meth-induced memory deficits in object recognition tests and restored the induction of pRh LTD.
MATERIALS AND METHODS
Surgery and SA
Surgical procedures and meth SA were performed as previously described (Reichel et al, 2014). Briefly, male Sprague Dawley rats were implanted with intracranial cannulae and/or jugular catheters in a single surgery. Following recovery, subjects self-administered meth hydrochloride (Sigma, St Louis, MO; dissolved in sterile 0.9% saline) in 1-h/day sessions for 7 days and then switched to 6-h/day access for 14 days. A response on the active lever resulted in a meth infusion (20 μg/50 μl bolus) and the 5 s presentation of a stimulus complex (light and tone). Yoked controls received saline whenever matched subjects received meth. All procedures were conducted in accordance with the ‘Guide for the Care and Use of Laboratory Rats’ (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011) and approved by the IACUC of the Medical University of South Carolina.
Novel Object Recognition
Novel object recognition (NOR) was performed as previously described (Reichel et al, 2011). In brief, rats were habituated to the test apparatus twice for 5 min without objects. On abstinence day 7, during familiarization, rats explored two identical objects for 3 min. A short-term memory test was conducted 90 min later by allowing rats to explore an object from the familiarization phase and a novel object for 3 min. A long-term test was conducted 24 h after sampling, in which rats were given the familiar object and a different novel object. Object exploration during both familiarization and NOR testing was defined as time spent sniffing or touching the object with the nose but not sitting, leaning, or standing on the object and was scored and reported as time spent with object (seconds; novel or familiar). Using these values, the recognition index was calculated by dividing the time spent with the novel object by the time spent with both objects. Approaches to the object were also quantified in order to calculate an approach score (approaches to novel object over total approaches). Familiar and novel objects, as well as the placement of the objects were counterbalanced. Objects consisted of combinations of a PVC pipe (6.4 × 3.8 cm2), a light bulb (8.9 cm), and a plastic bottle (Reichel et al, 2014). Total distance traveled in cm (ambulation) was also recorded and reported as a control measure.
Biotinylation and GluN2B/GluN1 Quantification
The surface biotinylation assay was performed as previously described (Knackstedt et al, 2014; Schwendt et al, 2012). In the aforementioned and present study, pRh tissue punches include a small amount of material from the neighboring entorhinal and ectorhinal cortices (Supplementary Figure S2). Briefly, 300 μm slices were made from 2 mm tissue punches of the pRh area with a tissue chopper and incubated with 1 mg/ml Sulfo-NHS-Biotin (Pierce) for 30 min at 4 °C with gentle shaking. The reaction was quenched by 100 mM glycine in PBS (pH 8.0). Tissue was washed in PBS and then sonicated in RIPA buffer. After incubating on ice, insoluble fragments were removed by centrifugation at 10 000 × g for 10 min at 4 °C. 500 μg of sample was combined with NeutrAvidin agarose resin and incubated for 2 h at 4 °C with rotation. A capillary-based size sorting system, ‘WES’ by ProteinSimple (Bio-Techne) was used to quantify protein levels as previously described (O'Neill et al, 2006). Briefly, prior to loading the WES plate, lysates (1 μg/μl stocks) were diluted to 0.2 μg/μl by combining 2.5 μl lysate, 2.5 μl master mix and 7.5 μl 0.1 × sample buffer for a total volume 12.5 μl. The reaction mix was then heated at 70 °C for 10 min. 5 μl of the reaction mix was then pipetted into the WES plate resulting in a final concentration of 1 μg in the capillary. Lysate mixture, blocking reagent, antibodies, chemiluminescent substrate, and wash buffer were then dispensed into designated wells on the WES microplate. Stacking matrix load time was set at 20 s and sample load time was set at 10 s. Data were analyzed using Compass software (ProteinSimple) using the ‘Gaussian fit’ function for quantitative analysis of GluN1, GluN2B, and Calnexin (see Figure 2c). The primary antibodies used were GluN2B (mouse, BD Biosciences, 610416, 1:500), GluN1 (mouse, BD Biosciences, 556308, 1:1000), and Calnexin (rabbit, Enzo Life Sciences, ADI-SPA-860-F, 1:2000). Surface (biotinylated) and intracellular (non-biotinylated) GluN2B signals were normalized to that of calnexin and expressed as a percent averaged saline values.
Microinjections and Histology
Injection cannulae (26 gauge, Plastics One) were positioned 1.5 mm above the pRh region via stereotaxic surgery at a 12° angle. pRh coordinates were −4.8 A/P, +5 M/L, −7.5 D/V. Although this cannulation targets the pRh, it is likely that drug solutions delivered may have been able to diffuse into the nearby ectorhinal and entorhinal cortices. DCS (Sigma) was dissolved in Milli-Q ultra-pure water at 20 mg/ml and rats were given either 0.5 μl/side of vehicle (Milli-Q water) or DCS (10 μg/side). Infusions were bilaterally administered through 33 gauge injectors (projected 1.5 mm past the guide cannula tip) at a flow rate of 0.2 μl/min. Cannula placement was determined following an overdose of Equithesin and perfusion with 4% formaldehyde and coronal slices (100 μm) were made for cresyl violet staining.
Field Recordings
Acute slices containing pRh, entorhinal, and temporal cortex were obtained from meth or yoked-saline rats as previously described (Cho et al, 2000). Briefly, brain tissue was rapidly removed, and midsaggital slices were cut at 400 μm thickness in ice-cold dissection artificial cerebrospinal fluid (aCSF) solution, using a previously described aCSF composition (Trantham-Davidson et al, 2014). Slices were incubated at 34 °C for at least 1 h before recordings in incubation aCSF. Slices were continuously aerated with 5% carbon dioxide/95% oxygen. After incubation, slices were transferred to a submerged recording chamber, held at 34 °C, and bathed with oxygenated recording aCSF. The pH of all solutions listed above was adjusted to 7.3 using NaOH and osmolarity was measured to be ~300 mOsm.
Extracellular field recordings were made with a Multiclamp 700B amplifier (Axon Instruments, Union City, CA), connected to a computer running Axograph X software and later analyzed offline. The recording electrode was filled with recording aCSF, had a resistance of <1 MΩ and was placed in the superficial layer of pRh, medial to the rhinal fissure. Two tungsten bipolar stimulating electrodes were placed in the superficial layers of the entorhinal and temporal cortices. Stimulation pulses were delivered every 30 s at 0.033 Hz and alternated between the regions using a Grass S88 stimulator and stimulus isolation unit. First, an input–output relationship was obtained by varying the intensity of stimulation from an amount that produced no field excitatory post-synaptic potential (fEPSP) up to an amount that produced a response of maximal amplitude, regardless of further increases in stimulus intensity. The intensity was then dialed down to generate a response that was ~75% of the maximal amplitude. Next, fEPSPs were recorded for 15 min to obtain a baseline amplitude measurement followed by application of low-frequency stimulation (LFS; 900 pulses at 1 Hz) to the entorhinal input previously shown to produce LTD in the pRh cortex (Cho et al, 2000; Ziakopoulos et al, 1999).
Statistics
Significant differences in time spent with objects during NOR testing, approach, and total ambulation were assessed using two-tailed t-tests. Significant differences in the WES protein quantification study were performed with one-tailed t-tests. Recognition indexes and field recordings were compared with theoretical means using one-sample t-tests. Meth infusions during extended access and GluN2B surface expression assays were analyzed using one-way ANOVA with Bonferroni post hoc analyses. Results were considered statistically significant at p<0.05. Sample sizes were chosen based on previous results from recognition memory tasks, protein quantification, and field recording studies. Sample exclusion criteria were determined prior to experimentation for technical failures, including failure of jugular catheters during meth SA. During field recordings, recognition memory testing, and WES protein quantification, the investigator was blind to group allocation during experimentation.
RESULTS
Meth SA
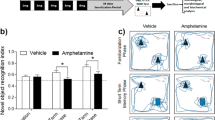
Meth subjects readily pressed the active lever for drug and increased responding during 6-h sessions. Yoked-saline controls indiscriminately sampled both levers (Figure 1a). Meth rats displayed escalating drug intake with significant increases in infusions across 6-h sessions (session effect (F(18,234)=15.38, p<0.0001) with higher intake on days 8–14 of 6-h access compared with the first day (Bonferroni’s multiple comparison, p<0.05; Figure 1b).
Extended access meth SA produced deficits in NOR. (a) Meth rats readily pressed the active lever for drug and increased responding during 6-h sessions. Yoked-saline controls indiscriminately sampled both levers. (b) Meth rats escalated drug intake, with significant increases in the number of infusions on days 8–14 of 6-h access compared with the first day (*p<0.05). (c) Time spent with each object during NOR is shown here with saline rats spending significantly more time with the novel object when compared with the familiar object (***p<0.001). (d) Recognition index for meth and yoked-saline controls. Yoked-saline controls displayed robust object recognition memory (+p<0.001). In contrast, recognition scores in meth rats did not significantly differ from chance exploration. Direct comparison revealed that meth recognition scores were significantly lower than yoked-saline controls (***p<0.001). Meth, methamphetamine; NOR, novel object recognition; SA, self-administration.
NOR
Following 1 week of abstinence, rats underwent NOR testing. During familiarization, yoked-saline and meth rats displayed similar time spent with either object (light bulb or plastic bottle), demonstrating that both groups of subjects equally explored both objects (Table 1A). Moreover, both groups showed no significant differences in the approach index (novel object/both objects) or total ambulation (cm) during familiarization (Table 1A).
During NOR testing, yoked-saline rats spent significantly longer time with the novel object (t(30)=5.373, p<0.0001; Figure 1c). Moreover, yoked-saline subjects displayed robust NOR memory (recognition index) compared with chance levels of exploration (a hypothetical mean of 0.5; t(16)=6.66, p<0.0001; Figure 1d). Direct comparison revealed that meth recognition scores were significantly lower than yoked-saline controls (t(29)=6.00, p<0.0001; Figure 1d). During NOR testing neither the approach scores (yoked-saline 0.54±0.03 and meth 0.46±0.02) nor ambulation values (distance traveled in cm; yoked-saline 2165±118; meth 2351±142) significantly differed between yoked-saline and meth groups (Table 1A).
GluN2B and GluN1 Expression
In order to test the hypothesis that chronic meth SA decreases surface expression of the NMDA receptor subunits GluN2B and GluN1, we selectively labeled surface receptors in pRh slices with biotin as previously described (Schwendt et al, 2012; Knackstedt et al, 2014). It is important to note that the pRh punches made for this study contain a small amount of material from the surrounding ectorhinal and entorhinal cortices (see Supplementary Figure S2). We observed a significant decrease in surface expression of GluN2B in rats with a history of extended access to meth (t(19)=1.891, p=0.037; Figure 2a). However, intracellular (non-biotinylated) GluN2B levels did not significantly differ. Although we observed no significant decrease in surface or intracellular expression of GluN1 in rats with a history of extended access to meth (Figure 2b), representative electropherograms used in the quantification of GluN2B by the WES system are shown in Figure 2c.
WES quantification of GluN2B and GluN1 surface expression. (a) Representative images of GluN2B immunoreactivity for both the surface (left) and intracellular fractions (right) in saline (S) and meth (M) subjects. We observed a significant decrease in surface expression of GluN2B in subjects with a history of extended access to meth (*p<0.05). Values obtained for intracellular levels GluN2B expression did not significantly differ between the meth and the yoked-saline groups. (b) Representative images of GluN1 immunoreactivity for both the surface (left) and intracellular fractions (right) in saline (S) and meth (M) subjects. Values obtained for surface or intracellular levels GluN1 expression did not significantly differ between the meth and the yoked-saline groups. (c) Representative electropherograms for GluN2B in the surface and intracellular fractions. Signals were quantified using the Gaussian quantification method. The shaded grey regions depict the area under the curves used for quantification of the calnexin, GluN2B and GluN1 signals. Meth, methamphetamine.
pRh LTD Following Extended Access to Meth
We hypothesized that a dysfunction of GluN2B-containing NMDA receptors in the pRh cortex leads to a loss of pRh LTD, which may account for the meth-induced loss of recognition memory in rats. To test this, we performed field recordings in the superficial layer of pRh cortex in acute slices obtained from yoked-saline and chronic meth rats. Recordings from chronic meth rats revealed a complete lack of LTD in the pRh cortex using the 1 Hz LFS protocol (the final 20 min of the recording were analyzed vs hypothetical mean of 100%; t(19)=0.77, p=0.45), whereas LTD was robustly elicited in saline controls (t(19)=31.95, p<0.0001; Figure 3a). The percent change in amplitude (normalized to baseline) was also significantly different when comparing the final 20 min of the meth and yoked-saline control recordings (t(38)=17.26, p<0.0001). There were no significant differences in fEPSP amplitude between the saline and meth groups during baseline (saline baseline 577.72±189.96 pA; saline post LTD 397.34 ±111.34 pA; meth baseline 459.78±94.92 pA; meth post LTD 480.59±134.28 pA. In addition, the temporal input pathway served as control stimulation with no changes in fEPSP amplitude observed in either treatment group over the length of the recording, suggesting that the fEPSP was stable over long recording times (data not shown).
DCS reversed meth-induced inhibition of pRh LTD. Field recordings are shown on the left with averaged activity over the final 20 min of each recording shown in bar graphs on the right (a, b). (a) 1 Hz LFS induced LTD in yoked-saline controls (+++p<0.001), whereas chronic meth SA rats show almost no change from baseline (***p<0.001 saline vs meth). Representative recordings before and after stimulation for both groups are shown below the graph. (b) In slices from meth rats, bath application of the NMDA receptor agonist DCS restored LTD following LFS (+++p<0.001). This effect was blocked by the specific GluN2B-containing NMDA receptor antagonist Ro 25-6981 (***p<0.001, DCS vs DCS+Ro 25-6981), suggesting that LFS-induced pRh LTD is dependent upon the activation of GluN2B-containing NMDA receptors. DCS, D-cycloserine; LFS, low-frequency stimulation; LTD, long-term depression; meth, methamphetamine; NMDA, N-methyl-D-aspartate; pRh, perirhinal.
pRh LTD and GluN2B Activation
In slices from meth rats, we bath applied the NMDA partial agonist DCS (100 μM), and found that LTD could be restored through DCS facilitation of GluN2A/2B-continaing NMDA receptors (t(19)=16.75, p<0.0001; Figure 3b). Co-application of DCS with Ro 25-6981 (3 μM), a potent and selective antagonist at GluN2B-containing NMDA receptors (Fischer et al, 1997), entirely prevented DCS-mediated restoration of LTD in chronic meth rats (DCS values were significantly greater than baseline; t(19)=8.72, p<0.0001). Further, percent change in amplitude was also significantly different when comparing the final 20 min of the DCS and DCS+Ro 25-6981 recordings (t(38)=17.93, p<0.0001; Figure 3b). These data confirm the importance of GluN2B-containing NMDA receptors in the DCS-restoration of LTD in chronic meth rats.
DCS Rescues Meth-Induced Deficits in NOR
We infused either vehicle or DCS (10 μg/side) bilaterally into the pRh in subjects that had undergone extended meth access followed by 1 week of abstinence, immediately following familiarization. During familiarization, vehicle and DCS rats spent similar amounts of time with each object, indicating equal exploration of the test apparatus (Table 1B). Moreover, both groups showed no significant differences in approach or ambulation during familiarization (Table 1B).
As described above, meth rats showed NOR memory deficits 90 min after familiarization, indicated by equal exploration of novel and familiar objects. However, rats that received bilateral pRh DCS infusions spent significantly more time with the novel object (t(12)=2.50, p=0.028). As expected, meth rats that received DCS infusions had recognition scores that were significantly higher than chance performance (t(6)=2.81, p=0.031), indicating restoration of NOR memory (Figure 4a). Direct comparison of vehicle and DCS groups revealed a significant increase in recognition scores for DCS-treated animals relative to controls (t(12)=3.73, p=0.003; Figure 4a). Importantly, during testing, neither approach nor ambulation differed between the vehicle and DCS groups (Table 1B).
DCS microinfusion into the pRh reversed meth-induced NOR deficits. (a) Meth rats, infused with vehicle, spent similar amounts of time with the familiar and novel object. However, infusion with DCS in the pRh produced a significant increase in time spent with the novel object (*p<0.05). Furthermore, meth rats showed significantly lower recognition scores when compared with chance levels of exploration, indicating object recognition memory deficits (+p<0.05). In contrast, meth rats that received bilateral perirhinal DCS infusions had recognition scores significantly higher than chance exploration (+p<0.05), indicating intact recognition memory. Direct comparison of meth/vehicle and meth/DCS groups also revealed a significant increase in recognition scores for DCS treated subjects relative to controls (**p<0.01). (b) 24 h later, meth rats that received vehicle still show equal amounts of time spent with each object, those that received DCS showing a trend toward increased time spent with the novel object. Meth rats that received vehicle also show recognition scores equivalent to chance levels, indicating object recognition memory deficits. In contrast, rats that previously received bilateral perirhinal DCS infusions had recognition scores significantly higher than chance exploration (+p<0.05). (c) DCS infusion sites are depicted in serial coronal planes (3.6–5.6 mm posterior to bregma). DCS, D-cycloserine; meth, methamphetamine; NOR, novel object recognition; pRh, perirhinal; SA, self-administration.
A long-term memory test for NOR was conducted at 24 h after the initial infusions of vehicle or DCS. Rats that had previously received DCS showed a nearly significant increase in time spent with the novel object (t(8)=2.22, p=0.0568), and showed recognition indexes that were significantly higher than a hypothetical mean of 0.5 (t(4)=2.88, p<0.05). These data indicate some recognition of the novel object even 24 h later. Despite the enduring enhancement of recognition memory in the DCS group, the recognition index did not significantly differ from that of the vehicle group (t(10)=1.31, p=0.22; Figure 4b). During this later test, neither the approach scores (vehicle 0.54±0.03; DCS 0.51±0.06), nor the ambulation values (vehicle 2804±258; DCS 2718±571) differed between the vehicle and DCS groups. Placements of cannulae used for infusion of vehicle or DCS into the pRh were verified ex vivo (Figure 4c).
In a series of control experiments, yoked-saline subjects were given bilateral DCS or vehicle microinfusions prior to NOR testing as described above. During familiarization, vehicle and DCS-treated yoked saline rats displayed no significant differences in time spent with objects, approach index or total ambulation (Table 1C). During NOR testing, both vehicle (t(14)=3.24, p=0.006) and DCS (t(12)=2.91, p=0.013) rats spent significantly more time with the novel object (Supplementary Figure S1A) and had recognition indexes that were significantly higher than chance exploration (vehicle: (t(7)=11.08, p<0.0001), and DCS: (t(6)=3.06, p=0.022); Supplementary Figure S1B).
DISCUSSION
Previous studies have shown that the pRh cortex has an important role in novelty detection, and indeed exposure to novel visual stimuli activates this region in humans (Pihlajamaki et al, 2003; Staresina et al, 2012). In support of human neuroimaging data, rodent studies show greater activation of pRh neurons following the perception of novelty (Brown and Aggleton, 2001). The current model of novelty encoding involves elevated pRh activity during the perception of novel objects, which gives way to LTD as familiarity develops (Montaldi et al, 2006; Barth and Wheeler, 2008). Here we report that extended access meth SA followed by 7 days of withdrawal impaired the ability to induce LTD in the pRh. These data support the hypothesis that meth rats have impaired NOR memory due to an inability to attribute familiarity to a particular object as a result of the loss of synaptic plasticity in the pRh. Although this has not been measured in human meth addicts using neuroimaging approaches, the data presented here suggest that the pRh may be a target area for future exploration in meth addicts.
Consistent with others (Cho et al, 2000), we show that NMDA receptors are required for the induction of pRh LTD (Supplementary Figure S3). Further, prior studies showed that blockade of GluN2B-containing NMDA receptors with Ro 25-6981 inhibits the induction of pRh LTD (Massey et al, 2004) and that pRh LTD is required for NOR (Griffiths et al, 2008). As such, we hypothesize that downregulation of pRh GluN2B-containing NMDA receptors following meth SA and withdrawal is responsible for the lack of pRh LTD and the subsequent inability to recognize novelty during NOR testing. It is also possible that extended access meth SA alters basal glutamate transmission in the pRh, which negatively impacts the ability to engage synaptic plasticity and induce LTD. A similar loss of LTD induction has been observed in the nucleus accumbens following cocaine SA (Martin et al, 2006; Moussawi et al, 2009; Huang et al, 2011). Loss of accumbal LTD occurs due to decreased expression of proteins that mediate homeostatic levels of extracellular glutamate, which acts to regulate synaptic plasticity. The consequent loss of glutamate tone ultimately leads to an inability to induce LTD (Moussawi et al, 2009), which can be mitigated by restoring tone on presynaptic metabotropic glutamate receptors (Moussawi et al, 2009; Kupchik et al, 2012). One alternative interpretation of the results presented here is that DCS treatment alters basal glutamate transmission to allow for the induction of pRh LTD.
We found that extended meth SA blocked both short-term and LTD and we were able to restore LTD with DCS but not short-term LTD. This suggests that meth exposure may have additional effects on calcium channels that are not sufficient to prevent LTD but could have other important consequences that will be examined in future studies.
Surface biotinylation experiments revealed that meth-induced disruption of LTD coincides with a diminished presence of surface GluN2B-containing NMDA receptors in the pRh cortex. In the current study, we did not investigate the mechanism of GluN2B-containing NMDA receptor internalization or the fate of internalized receptors. However, recent evidence suggests that psychostimulant-induced internalization of GluN2B-containing NMDA receptors can be caused by proteasomal degradation of GluN2B-anchoring proteins (Mao et al, 2009). In the present study, the decrease in GluN2B-containing NMDA receptors at the surface was not accompanied by an increase in the intracellular pool. It is possible that receptor degradation may explain this discrepancy. As the blockade of GluN2B degradation improves synaptic plasticity and memory (Hawasli et al, 2007; Lee and Silva, 2009), future studies will investigate whether the persistent character of pRh LTD and memory deficits following meth exposure is related to internalization and degradation of local GluN2B-containing NMDA receptors.
As a non-selective partial agonist of NMDA receptors, DCS does not distinguish between GluN2A- or GluN2B-containing NMDA receptors. We chose DCS to verify our findings from physiological analyses of pRh LTD in the NOR behavioral model due to the fact that no selective agonists of GluN2B-containing NMDA receptors are suitable for microinjection in the brain. Despite the fact that the DCS reversal of meth-induced pRh LTD impairment was prevented by administration of a specific GluN2B antagonist, Ro 25-6981 (Figure 3b), it is important to note that we cannot conclude from the DCS microinfusion experiments that restoration of NOR occurred through a GluN2B-dependent mechanism, only that NOR memory was restored through the non-selective activation of pRh NMDA receptors.
The induction of NMDA-dependent LTD requires receptor activation, calcium influx, activation of calcineurin and the internalization of AMPA receptors (Beattie et al, 2000). Accordingly, direct inhibition of AP2-mediated AMPA receptor internalization disrupts both pRh LTD and NOR memory (Griffiths et al, 2008). As such, down regulation of GluN2B-containing NMDA receptors in the pRh following chronic meth SA may also impact AMPA receptor internalization through this mechanism. Further investigation is required to determine whether the downregulation of GluN2B-containing NMDA receptors and dysregulation of AMPA receptor internalization is indeed the primary mechanism for both the loss of pRh LTD and NOR memory impairment following chronic meth SA.
Here, we demonstrate that extended access meth SA followed by withdrawal resulted in NOR memory deficits and impaired the ability to induce pRh LTD. These deficits are likely the consequence of the downregulation of GluN2B-containing NMDA receptors in the pRh. Further, DCS restored pRh LTD in meth subjects in a GluN2B-dependent manner. Finally, DCS microinfusion into the pRh reversed deficits in NOR memory, consistent with its ability to restore pRh LTD. In light of these data, we conclude that the meth-induced deficit in NOR memory is likely due to the loss of pRh LTD. Our data indicate that the pRh may be a key brain region disrupted by chronic meth exposure. Given that relapse probability is related to episodic memory impairments in meth addicts (Simon et al, 2004), these data suggest that pRh LTD can be targeted to restore cognition in meth addicts.
Funding and Disclosure
The authors declare no conflict of interest.
References
Albasser MM, Davies M, Futter JE, Aggleton JP (2009). Magnitude of the object recognition deficit associated with perirhinal cortex damage in rats: effects of varying the lesion extent and the duration of the sample period. Behav Neurosci 123: 115–124.
Barker GR, Bird F, Alexander V, Warburton EC (2007). Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci 27: 2948–2957.
Barker GR, Warburton EC (2008). NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. J Neurosci 28: 2837–2844.
Barker GR, Warburton EC (2011). When is the hippocampus involved in recognition memory? J Neurosci 31: 10721–10731.
Barth AL, Wheeler ME (2008). The barista on the bus: cellular and synaptic mechanisms for visual recognition memory. Neuron 58: 159–161.
Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M et al (2000). Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3: 1291–1300.
Brown MW, Aggleton JP (2001). Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci 2: 51–61.
Cho K, Kemp N, Noel J, Aggleton JP, Brown MW, Bashir ZI (2000). A new form of long-term depression in the perirhinal cortex. Nat Neurosci 3: 150–156.
Dean AC, Groman SM, Morales AM, London ED (2013). An evaluation of the evidence that methamphetamine abuse causes cognitive decline in humans. Neuropsychopharmacology 38: 259–274.
Ennaceur A (2010). One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215: 244–254.
Fischer G, Mutel V, Trube G, Malherbe P, Kew JN, Mohacsi E et al (1997). Ro 25-6981, a highly potent and selective blocker of N-methyl-D-aspartate receptors containing the NR2B subunit. Characterization in vitro. J Pharmacol Exp Ther 283: 1285–1292.
Griffiths S, Scott H, Glover C, Bienemann A, Ghorbel MT, Uney J et al (2008). Expression of long-term depression underlies visual recognition memory. Neuron 58: 186–194.
Hart CL, Marvin CB, Silver R, Smith EE (2012). Is cognitive functioning impaired in methamphetamine users? A critical review. Neuropsychopharmacology 37: 586–608.
Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P et al (2007). Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci 10: 880–886.
Huang CC, Yeh CM, Wu MY, Chang AY, Chan JY, Chan SH et al (2011). Cocaine withdrawal impairs metabotropic glutamate receptor-dependent long-term depression in the nucleus accumbens. J Neurosci 31: 4194–4203.
Kealy J, Commins S (2011). The rat perirhinal cortex: a review of anatomy, physiology, plasticity, and function. Prog Neurobiol 93: 522–548.
Knackstedt LA, Trantham-Davidson HL, Schwendt M (2014). The role of ventral and dorsal striatum mGluR5 in relapse to cocaine-seeking and extinction learning. Addict Biol 19: 87–101.
Kupchik YM, Moussawi K, Tang XC, Wang X, Kalivas BC, Kolokithas R et al (2012). The effect of N-acetylcysteine in the nucleus accumbens on neurotransmission and relapse to cocaine. Biol Psychiatry 71: 978–986.
Lee YS, Silva AJ (2009). The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci 10: 126–140.
Malkova L, Forcelli PA, Wellman LL, Dybdal D, Dubach FM, Gale K (2015). Blockade of glutamatergic Transmission in perirhinal cortex impairs object recognition memory in macaques. J Neurosci 35: 5043–5050.
Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ et al (2009). Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci 12: 602–610.
Marshall JF, O'Dell SJ (2012). Methamphetamine influences on brain and behavior: unsafe at any speed? Trends in neurosciences 35: 536–545.
Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A (2006). Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci 9: 868–869.
Massey PV, Johnson BE, Moult PR, Auberson YP, Brown MW, Molnar E et al (2004). Differential roles of NR2A and NR2B-containing NMDA receptors in cortical long-term potentiation and long-term depression. J Neurosci 24: 7821–7828.
Massey PV, Phythian D, Narduzzo K, Warburton EC, Brown MW, Bashir ZI (2008). Learning-specific changes in long-term depression in adult perirhinal cortex. J Neurosci 28: 7548–7554.
McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J et al (2008). Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse 62: 91–100.
Montaldi D, Spencer TJ, Roberts N, Mayes AR (2006). The neural system that mediates familiarity memory. Hippocampus 16: 504–520.
Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A et al (2009). N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci 12: 182–189.
O'Neill RA, Bhamidipati A, Bi X, Deb-Basu D, Cahill L, Ferrante J et al (2006). Isoelectric focusing technology quantifies protein signaling in 25 cells. Proc Natl Acad Sci USA 103: 16153–16158.
Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V et al (2003). Encoding of novel picture pairs activates the perirhinal cortex: an fMRI study. Hippocampus 13: 67–80.
Reichel CM, Gilstrap MG, Ramsey LA, See RE (2014). Modafinil restores methamphetamine induced object-in-place memory deficits in rats independent of glutamate N-methyl-d-aspartate receptor expression. Drug Alcohol Depend 134: 115–122.
Reichel CM, Ramsey LA, Schwendt M, McGinty JF, See RE (2012). Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62: 1119–1126.
Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE (2011). Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology 36: 782–792.
Reichel CM, See RE (2010). Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology (Berl) 210: 337–346.
Rogers JL, De Santis S, See RE (2008). Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology (Berl) 199: 615–624.
Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH (2009). Drug abstinence and cognitive control in methamphetamine-dependent individuals. J Subst Abuse Treat 37: 292–297.
Schwendt M, Reichel CM, See RE (2012). Extinction-dependent alterations in corticostriatal mGluR2/3 and mGluR7 receptors following chronic methamphetamine self-administration in rats. PLoS One 7: e34299.
Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH et al (2007). Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev 17: 275–297.
Simon SL, Dacey J, Glynn S, Rawson R, Ling W (2004). The effect of relapse on cognition in abstinent methamphetamine abusers. J Subst Abuse Treat 27: 59–66.
Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN (2012). Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nat Neurosci 15: 1167–1173.
Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW et al (2014). Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J Neurosci 34: 3706–3718.
Warburton EC, Barker GR, Brown MW (2013). Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74: 41–47.
Wood S, Sage JR, Shuman T, Anagnostaras SG (2014). Psychostimulants and cognition: a continuum of behavioral and cognitive activation. Pharmacol Rev 66: 193–221.
Ziakopoulos Z, Tillett CW, Brown MW, Bashir ZI (1999). Input-and layer-dependent synaptic plasticity in the rat perirhinal cortex in vitro. Neuroscience 92: 459–472.
Acknowledgements
We thank Shannon Ghee, Nastassia Bryant, and Sarah Barry for technical assistance. This research was supported by National Institute on Drug Abuse (NIDA) grants DA033049, DA007288, and NIH grant C06 RR015455.
Author Contributions
All authors discussed the experimental design, results and implications, and commented on the manuscript. MDS, JP, and CMR contributed to and conducted the behavioral components. MDS, MS, K-CL, and CMR contributed to and conducted the neurochemistry component. HT-D conducted, analyzed, and wrote the electrophysiology part of this work. MDS, HT-D, and CMR wrote the manuscript. JP, RES, and MS gave technical support and conceptual advice, as well as reviewed and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Scofield, M., Trantham-Davidson, H., Schwendt, M. et al. Failure to Recognize Novelty after Extended Methamphetamine Self-Administration Results from Loss of Long-Term Depression in the Perirhinal Cortex. Neuropsychopharmacol 40, 2526–2535 (2015). https://doi.org/10.1038/npp.2015.99
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.99
This article is cited by
-
Repeated methamphetamine administration produces cognitive deficits through augmentation of GABAergic synaptic transmission in the prefrontal cortex
Neuropsychopharmacology (2022)
-
Current understanding of methamphetamine-associated dopaminergic neurodegeneration and psychotoxic behaviors
Archives of Pharmacal Research (2017)
-
Methamphetamine self-administration modulates glutamate neurophysiology
Brain Structure and Function (2017)
-
Perirhinal Cortex mGlu5 Receptor Activation Reduces Relapse to Methamphetamine Seeking by Restoring Novelty Salience
Neuropsychopharmacology (2016)