Abstract
A number of studies have shown an association between diabetes and depression. However, the underlying mechanisms are still unclear. Previous findings indicate a role for the prefrontal cortex and subcortical gray matter regions in type 2 diabetes and major depressive disorder (MDD). The purpose of this study was to examine the white matter integrity in the fibers that are part of the anterior limb of internal capsule (ALIC) in MDD and diabetic subjects using diffusion tensor imaging tractography. We studied 4 groups of subjects including 1) 42 healthy controls (HC), 2) 28 MDD subjects (MD), 3) 24 patients diagnosed with type 2 diabetes without depression (DC), and 4) 22 patients diagnosed with diabetes and depression (DD). Results revealed significantly decreased fractional anisotropy (FA; P=.021) and a trend towards significant increase in radial diffusivity (RD; P=.078) of the right ALIC in depressed subjects (MD+DD) compared to non-depressed subjects (HC+DC). While there were no significant diabetes effects or interactions between depression and diabetes, subjects with high depression ratings and high hemoglobin A1c levels had the lowest mean FA values in the right ALIC. In addition, we found a significant negative correlation between FA of the left ALIC with hemoglobin A1c in diabetic subjects (DC+DD; P=.016). Our study demonstrated novel findings of white matter abnormalities of the ALIC in depression and diabetes. These findings have implications for clinical manifestations of depression and diabetes as well as their pathophysiology.
Similar content being viewed by others
INTRODUCTION
The interaction between type 2 diabetes and major depression has long been observed and extensively studied (Leonard et al, 2002; Anderson et al, 2001; Nichols et al, 2003). Individuals with diabetes were twice as likely as a comparable sample to be diagnosed with comorbid depression (estimated at around 15% to 20% in patients with diabetes). It has been hypothesized that several neuroendocrine and neuroinflammatory processes contribute to the underlying pathophysiology (Rustad et al, 2011), but the neuroanatomical correlates are less clear.
Across several studies, our group has found distinct subcortical alterations associated with depression and diabetes. For example, using magnetic resonance imaging (MRI) magnetization transfer (MT) technique, we have previously reported significantly lower MT ratios (MTR) in the head of the caudate nuclei bilaterally in participants with both diabetes and major depression (MDD) compared to healthy controls or individuals with diabetes alone (Kumar et al, 2009). Using proton MRS (magnetic resonance spectroscopy), we further found glutamine and glutamate concentrations in depressed diabetic subjects to be significantly lower in select subcortical regions including the caudate as compared to healthy and diabetic control subjects. Furthermore, myo-inositol concentrations were significantly increased in depressed diabetic subjects as well as diabetic control subjects within the frontal white matter (WM) when compared to healthy controls (Ajilore et al, 2007). Additionally, a recent study by Hsu et al found significantly decreased fractional anisotropy (FA) values in the bilateral frontal WM in type 2 diabetic subjects compared to healthy controls and a significant association between disease duration and microstructural properties in several brain regions including bilateral cerebellum, temporal lobe WM, right caudate, bilateral cingulate gyrus, pons, and parahippocampal gyrus (Hsu et al, 2012).
The caudate nucleus and prefrontal white matter region have been identified as important components of cortico-striatal-pallidal-thalamic (CSPT) circuits that are thought be disrupted in major depression. These circuits are involved in the complex regulation of motor functions, cognition, and mood (Alexander et al, 1990). Among the neuroanatomical abnormalities in these circuits that are associated with major depression are decreased cortical and subcortical volumes, white matter hyperintensities and impaired white matter integrity (Kumar and Cook 2002; Krishnan et al, 2006; Alexopoulos et al, 2008; Kempton et al, 2011; Bora et al, 2011).
In this paper, we were interested in examining the white matter microstructural integrity of a key component in CSPT circuits in both MDD and diabetes. Specifically, we studied fibers in the anterior limb of the internal capsule (ALIC) which contains the anterior thalamic radiation (ATR), prefrontal corticopontine tracts, thalamo-striate and striate-striate tracts (Kahle et al, 2002; Axer and Keyserlingk 2000; Cunningham 1903). Fibers in the ALIC are directed horizontally, obliquely laterally, and upward toward the frontal lobe and in horizontal sections of the hemisphere they appear to be cut longitudinally (Carpenter, 1991). Previous studies of the ALIC region in MDD have shown decreased white matter volume in treatment-resistant depression, decreased FA in depressed subjects and more specifically, suicide attempters (Philips et al, 2012; Zou et al, 2008; Jia et al, 2010; Zhu et al, 2011).
In addition to standard diffusivity measures of white matter integrity, such as FA and mean diffusivity, we explored two additional measures including the axial diffusivity (the degree of water diffusion along the direction parallel to the fiber bundles) and the radial diffusivity (water diffusion perpendicular to the axonal wall). These two measures may provide additional information on the relative diffusivity of water molecules beyond standard FA, and have been utilized to differentiate among various white matter microstructural changes (Song et al, 2003). Specifically, a decrease of axial diffusivity may reflect axonal injury while an increase of radial diffusivity may indicate a dysmyelination process (Song et al, 2002).
In this paper, we hypothesized that white matter abnormalities will be present in ALIC tracts of subjects with both MDD and diabetes when compared to healthy controls, with intermediate alterations for either disease state alone. We also hypothesized that these white matter integrity alterations will be negatively correlated with severity of MDD and diabetes.
MATERIALS AND METHODS
Subject Recruitment
Four groups of subjects were recruited from the greater Chicago area through flyers and local advertisements: 1) healthy control (HC); 2) depressed (MD); 3) diabetic (DC); 4) diabetic depressed (DD). This four-group design allows us to study the independent depression and diabetes effects on white matter integrity as well as any possible interaction. The inclusion criteria for all subjects were 30 years of age and older, medication-naive or anti-depressant free for at least two weeks and no history of unstable cardiac or neurological diseases. The exclusion criteria included: schizophrenia, bipolar or any psychotic disorders; history of anxiety disorder outside of major depressive episodes; history of head trauma; history of substance abuse other than alcohol or marijuana. Subjects with a history of alcohol or marijuana use greater than or equal to 5 years prior to study entry were acceptable. This study was approved by the University of Illinois-Chicago Institutional Review Board, and written informed consent was obtained from each participant.
All eligible subjects were assessed by a trained research assistant with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (First et al, 1997). The severity of depression was quantified by a board-certified/board-eligible psychiatrist (AK or OA) using the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton 1967). At the time of enrollment, MD and DD subjects met DSM-IV criteria for MDD and required a score of 15 or greater on the HAM-D. Subjects were also administered the Center for Epidemiologic Studies of Depression (CES-D) scale as an independent measure of depression severity (Radloff 1977). DD subjects had no more than two major depressive episodes prior to the onset of diabetes. The CES-D was used for correlation analyses as the HAM-D was the measure used in the determination of subject eligibility for depression. All diabetic participants were on oral hypoglycemic and/or insulin (Please see Supplementary Table 1 for medications classes used by all subjects). The diagnosis of type 2 diabetes was confirmed by anti-diabetic medications or elevated hemoglobin a1c (hgb A1c) levels.
MRI Image Acquisition
All brain MRI data were acquired using a Philips Achieva 3T scanner (Philips Medical Systems, Best, the Netherlands) with 8-element SENSE (Sensitivity Encoding) head-coil. Subjects were fitted with soft ear plugs, positioned comfortably in the coil, and instructed to remain still. Foam pads were used to minimize head motion. For each subject, DTI images were acquired using single-shot spin-echo echo-planar imaging (EPI) sequence (field of view or FOV=240mm; acquired voxel size =2.21 × 2.21 × 2.20 mm; reconstructed voxel size =0.83 × 0.83 × 2.2 mm; TR/TE=6,994/71 ms; Flip angle=90o). Sixty seven contiguous axial slices aligned to the anterior commissure–posterior commissure (AC-PC) line were collected along 32 gradient directions with b=700 s/mm2 and one minimally diffusion-weighted scan (the b0 image). Parallel imaging was utilized with p-factor of 2.5 to reduce scan time to approximately 4 min.
Image Processing
We carefully inspected all DTI image data to ensure quality, and 9 subjects with serious artifacts from substantial head movements were removed from the analysis. All diffusion weighted images (32 gradient directions) were first coregistered onto the b0 images by using the automatic image registration (AIR) algorithm with affine transformation to minimize eddy currents and remove any potential small bulk motions that occurred during the scans (Zhang et al, 2012; Woods et al, 1998; Woods et al, 1998). The diffusion directions in the diffusion gradient table were transformed by the rotation matrix calculated by AIR, to correct changes in section angulation due to coregistration. Diffusion tensor calculation and fiber tracking were then carried out using the DtiStudio software (Jiang et al, 2006) (Laboratory of Brain Anatomical MRI, Johns Hopkins Medical Institute, Baltimore). At each voxel, the signals from the 32 diffusion-weighted images were fitted to obtain the six elements of the diffusion tensor. The diffusion tensors were then diagonalized to obtain three eigenvalues (λ1, λ2, λ3) and three eigenvectors (v1, v2, v3). The eigenvector (v1) associated with the largest eigenvalue (AD: axial diffusivity) was assumed to represent the local fiber direction. Radial diffusivity (RD) is the mean of two minor eigenvalues (λ2+λ3)/2. To quantify the relative degree of anisotropy in a voxel, fractional anisotropy (FA) is calculated by the square root of the sum of squares (SRSS) of the diffusivity differences, divided by the SRSS of the diffusivities.
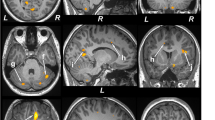
Fiber tracking was performed with the fiber assignment by continuous tractography (FACT) method (Mori and van Zijl, 2002). For each subject, tractography was first performed with brute-force tracking on the whole brain by initiating tracts at each voxel. Fiber tracking was stopped when FA value falls below 0.15 or a turning angle becomes larger than 60°. To obtain the ALIC tract, we selected the most superior axial slice where the genu of the internal capsule was most clearly identified. Then, the ALIC region was carefully traced and delineated from the junction of internal and external capsules to the lateral tip of genu. Virtual dissection was conducted in order to remove fibers not consistent with known human neuroanatomy (eg, fibers that traced beyond ATR or cortico-pontine tracts; fibers in the contralateral hemisphere). Figure 1 demonstrates sample tract profiles superimposed on the corresponding coregistered MPRAGE image in a sagittal view. Intra-rater and inter-rater reliabilities of fiber extraction were performed on a subset of 10 tracts (the bilateral ALIC fibers from 5 randomly chosen subjects) and 97.2% intra-rater agreement [ICC=0.972, P<0.001] and 80.0% inter-rater agreement [ICC=0.800, P=0.013] were reached for the FA values of all the tracts. To ensure consistency in extracting fiber tracts, all reconstructed fibers were visually inspected for quality assurance by a board-certified psychiatrist and experienced brain imaging researcher (AL).
(a) Slice selection displayed on MPRAGE image; (b) Regions of interest drawn on the corresponding color map for left and right ALIC; (c) Left ALIC visualization superimposed on sagittal MPRAGE image; (d) Right ALIC visualization superimposed on sagittal MPRAGE image.
For white matter hyperintensity (WMH) measurements, FLAIR images were loaded into the software package Jim (version 6.0, Xinapse Systems, Northants, UK; http://www.xinapse.com), and WMH were outlined using the contour function. All areas of increased signal were included unless: (i) the area was less than 5 mm3, (ii) the area was a narrow band along the ventricles and (iii) hyperintensities were due to the presence of blood vessels. Since WMH volumes (in mm3) were not normally distributed, the data was normalized using a natural-log transformation for statistical analysis.
Quantitative Tract-specific Measures
After fiber tracts are obtained, tract-specific measures were computed along the coordinates of the tracts to quantify white matter characteristics. The measures included mean FA, axial diffusivity (AD), radial diffusivity (RD) and mean diffusivity (MD). Mathematically for each tract, these measures were extracted along the voxels that the fibers travel through and weighted by the number of reconstructed fibers in the particular voxel. All these values were averaged from all reconstructed fibers in the ALIC to obtain a single number for each tract.
Statistical Analyses
All statistical analyses were performed in the SPSS software, version 18.0 (SPSS, Somers, NY). Clinical and demographic differences were analyzed using a one-way analysis of variance (ANOVA) for continuous variables and Pearson Chi-Square for categorical variables. Individual two-way analyses of covariance (ANCOVA) were used to assess group differences in all tract measures using the diagnosis of diabetes, the diagnosis of depression, and their interaction as factors. Post-hoc pairwise comparisons of all four subject groups for significant factors were also examined. Given the reported impact of age and sex on neuroanatomical measurements across various neuroimaging modalities (Barnes et al, 2010), these variables were used as initial covariates. Given our prior hypothesis, we conducted one-tailed Pearson’s product moment correlations to further explore the relationship between the depression severity scale CES-D and DTI measures in depressed subjects. These analyses were also controlled for age and sex. We did not perform correlation with HAM-D as it was used in our inclusion criteria. Correlation with hgb A1c was performed on FA measures of both tracts using one-tailed Pearson's correlation for the diabetic subjects. As an exploratory analysis, we examined differences in tract measures in four groups defined by a median split in depression severity and diabetes severity measures.
RESULTS
Demographic and Clinical Data
Table 1 summarizes the demographic and clinical characteristics of the subjects in four groups. There were no significant group differences in age, sex, years of education, blood pressure, body mass index (BMI) or white matter hyperintensity volumes. There was a significant difference in random high-density lipoprotein (HDL) levels with diabetic subjects (DC+DD) having lower levels compared to non-diabetic subjects (HC+MD). However, there were no significant differences between diabetic groups. As expected, there were significant differences across measures of depression (CES-D and HAM-D) and diabetes (hgb A1c and diabetes duration). No differences were found between DD and MD subjects in terms of depression severity. There were no pair-wise differences in hgb A1c or diabetes duration between DD and DC subjects. In addition, there were no significant differences in the frequency of microangiopathy-related diabetic complications between DC and DD subjects (neuropathy: 6 DC/8 DD, nephropathy: 2 DC/4 DD, and retinopathy: 1 DC/2 DD).
Between-group Comparison
2 × 2 ANCOVA revealed a significant depression effect on FA of the right ALIC [F(1,1)=5.5, P=0.021] (Table 2A, Figure 2a). Within the right ALIC, the radial diffusivity had a trend towards significant increase in the depressed cohort [MD+DD, F(1,1)=3.16, P=0.078] while axial diffusivity remained unchanged [F(1,1)=0.26, P=0.61]. No significant between-group differences were detected for mean diffusivity. Post-hoc pair-wise comparisons found the depression effect was primarily driven by HC and DD differences (P=0.033) compared to HC and MD differences (P=0.106). The strongest effect size was for the right ALIC FA difference between HC and DD subjects (Table 2B).
Scatter plots of FA values by group: (a) right ALIC; (b) left ALIC.
2 × 2 ANCOVA also revealed a trend toward significant diabetes effect on FA of the left ALIC [F(1,1)=3.14, P=0.079] (Figure 2b). Within the left ALIC, no significant between-group differences were detected for the axial, radial and mean diffusivities. There were no significant second order interactions between the covariates (age and sex).
Post-hoc analyses revealed that FA and radial diffusivity in the right ALIC were negatively correlated [r=−0.770, P<0.0001] in all subjects. Furthermore, the use of either one of the two measures as a covariate eliminated the depression factor group difference in the other measure for the right ALIC, suggesting a single physiological process responsible for FA and radial diffusivity changes. FA and axial diffusivity in the right ALIC were also negatively correlated across all subjects [r=−0.386, P<0.0001], but significantly less so (P<0.0001). In addition, adding axial diffusivity as a covariate did not eliminate the depression factor group difference.
FA was also negatively correlated with WMH volume (r=−0.255, P=0.007, df=110) in the right ALIC. However, adding WMH as a covariate, did not change the depression effect which remained a significant factor for the right ALIC [F(1,1)=4.73, P=0.032] and diabetes had a trend towards significance for the left ALIC [F(1,1)=3.43, P=0.067].
Since HDL levels were significantly different between groups and HDL has been associated with decreased regional white matter integrity in the internal capsule (Williams et al, 2012), the ANCOVA was repeated including HDL levels as an additional covariate. The significant depression effect on FA in the right ALIC remained [F(1,1)=7.63, P=0.007]. However, there was now a significant diabetes effect on FA in the left ALIC [F(1,1)=4.01, P=0.048] (Figure 2b).
Correlations of Tract Measures to Depression Severity
We further investigated the relationship between depression severity and tract-specific measures of the right ALIC in the MD and DD groups. Partial Pearson's correlation controlled for age and sex were performed between CES-D total score and both FA and RD. Within the MD group in the right ALIC, neither the FA [r=−0.308, P=0.134] nor RD [r=0.342, P=0.094] correlated with CES-D total score. These correlations were not significant in the DD subjects [FA: r=−0.045, P=0.854; RD: r=−0.032, P=0.896].
Correlations of Tract Measures to Diabetes Severity
In our exploratory partial correlation analysis (controlled for age and sex) with hgb A1c and both FA and RD in diabetic subjects (DC+DD), we found a significant correlation with the left ALIC FA [r=−0.360, P=0.016] and a trend towards significance in RD [r=0.261, P=0.088] but not in the right ALIC [r=−0.179, P=0.244]. Within the DC group, FA of the left ALIC correlated with hgb A1c [r=−0.436, P=0.043] but not RD [r=0.249, P=0.264]. Within the DD group, RD of the right ALIC correlated [r=0.486, P=0.030] but not FA [r=−0.301, P=0.197].
Influence of Alcohol and Marijuana Abuse History
While we excluded any subjects with current substance or alcohol abuse, there were 15 subjects with a history of marijuana and/or alcohol abuse (⩾5 years prior to study entry). To examine the influence of these subjects, we re-analyzed the data without them. Without these subjects, the 2 × 2 ANCOVA revealed that the right ALIC FA was reduced in depressed subjects compared to non-depressed subjects (0.448±.027 vs 456±.023), however it was no longer significant (P=0.078). Hgb A1c was still significantly correlated with the left ALIC FA [r=−0.362, P=0.02] among all diabetic subjects.
The Effect of Diabetes Severity on Depression Differences
Since the effect size for HC vs. DD differences were larger but not statistically significant and diabetic subjects represent varying levels of severity (defined by hgb A1c levels), an exploratory analysis was done to examine the role of diabetes severity on depression differences in FA of the right ALIC. Using a median split for depression severity (defined by CES-D scores above or below 16) and diabetes severity (hgb A1c below or above 5.8), subjects were divided into four groups. The four severity groups were closely matched to original diagnostic groups. 91% of the low diabetes/low depression group was HC, 82% of the low diabetes/high depression group was MD, 67% of the high diabetes/low depression was DC, and 63% of the high diabetes/high depression was DD. There was a significant difference in right ALIC FA values between low and high depression severity subjects only when hgb A1c levels were greater than 5.8 [F(1,1)=8.38, P=0.006] (Figure 3).
Effect of diabetes severity vs. depression severity on FA values of the right ALIC. Blue (low CES-D scores <16), green (high CES-D scores ⩾16).
DISCUSSION
In this study, we investigated tract-specific white matter alterations of ALIC in both depressed and diabetic subjects utilizing DTI-tractography based diffusivity measures. The main finding of our study showed that white matter integrity in the right ALIC is significantly impaired in depressed subjects compared to non-depressed subjects as measured by FA. This finding of decreased integrity of the ALIC in depression complements the growing literature demonstrating white matter alterations in the prefrontal cortex in late-life depression (Taylor et al, 2001; Bae et al, 2006; Shimony et al, 2009; Mettenburg et al, 2012). The decrease of FA in the right ALIC may be explained by a trend towards significant increase in the radial diffusivity (an index of myelin integrity), without significant alterations in axial diffusivity. As concluded in the pioneering work of Song et al, (Song et al, 2003; Song et al, 2002), a decrease of axial diffusivity may reflect axonal injury while an increase of radial diffusivity may indicate a dysmyelination process. Thus, our results (unchanged axial but increased radial diffusivity) suggested that the white matter impairment of the right ALIC may be related to dysmyelination in nature. This dysmyelination process could be due to inflammation or vascular damage as discussed in our previous work (Kumar and Cook, 2002). Alternatively, it is also possible that the local fiber architecture becomes less coherent, resulting in an artificial drop-off of FA values and an increase of radial diffusivity.
The link between impaired myelination and depression has been described in a number of neurological conditions characterized by white matter pathology including multiple sclerosis (MS). Since myelin can provide a 100-fold increase in action potential transmission speed, demyelinating conditions like MS can have a significant impact on the brain’s information processing capabilities (Bartzokis, 2012) and lead to cognitive and behavioral disturbances (Brassington and Marsh, 1998). Although demyelination in MS is a widespread process involving multiple brain regions, impaired white matter integrity in selected key tracts like the ALIC have been detected in MS (Ciccarelli et al, 2001; Tillema et al, 2012) and may underlie the increased risk of major depression.
The FA reduction in the right ALIC implicates that the brain regions connected via ALIC could contribute to the impairment of the white matter integrity, which confirms our earlier results in caudate nuclei using MT and MRS techniques (Kumar et al, 2009; Ajilore et al, 2007). Decreased FA in the ALIC has also been reported in a number of previous studies (Jia et al, 2010; Zhu et al, 2011). However, the left ALIC was implicated in those reports as opposed to the right ALIC in the present study. Different methodological techniques might explain the discrepancy. Those previous studies used voxel-based techniques such as tract-based spatial statistics (TBSS) and statistical parametric mapping (SPM), in contrast to our tract-specific approach which reduces the confound of fiber mixing and crossing that can occur in the ALIC. It is likely that both the left and right ALIC exhibit white matter alterations. Further evidence for the bilateral involvement of the ALIC is derived from deep brain stimulation (DBS) studies. Bilateral stimulation of the ALIC in MDD patients has been shown to induce acute improvements in mood (Machado et al, 2009). In addition, a tractography analysis of the bilateral ALIC as a DBS target revealed common networks with the subgenual cingulate, another region important in mood regulation (Gutman et al, 2009).
The trend towards significant diabetes effect in the left ALIC implicates that the diabetes condition contributes to the white matter impairment in the left side of the ALIC. Even though WMH significantly correlated with FA and thus may be a confound, this trend remained even after adding WMH as a covariate. This finding complements previous studies on frontal WM impairments in diabetes (Ajilore et al, 2007, Hsu et al, 2012). For example, Hsu et al found lower FA values in bilateral frontal WM in type 2 diabetic subjects compared to healthy controls. Furthermore, the significant negative correlation between diabetes severity and FA of the left ALIC agrees with the finding from the same study where they found a significant association between disease duration and microstructural properties in several brain regions including bilateral cerebellum, bilateral cingulate gyrus and pons (Hsu et al, 2012). The involvement of the left ALIC in diabetes and the right ALIC in depression suggest that patients with both diabetes and depression are more likely to have bilateral white matter integrity impairment compared to subjects with either disease alone. This implies that while there may not be a statistical interaction between diabetes and depression, there may be additive effects.
The lack of a significant interaction may be due to the limitation of the relatively small sample size in our diabetic subjects relative to non-diabetic subjects. Also suggestive of the influence of sample sizes was the finding of smaller effect sizes for DC comparisons to depressed subjects compared to HC subjects. When examining diabetes severity, FA differences in the right ALIC due to depression were more prominent with higher hgb A1c levels. In our exploratory correlation analysis in the combined diabetic subjects (DC+DD), we found FA of the left ALIC correlated with hgb A1c. This implies that while there were no overall significant diabetes effects detected, white matter integrity in the ALIC was nevertheless sensitive to diabetes severity and may be due to the additive effects of diabetes and depression.
Another limitation of the study was the inclusion of subjects with a history of alcohol and marijuana abuse in our sample. Alcohol and marijuana abuse are very common co-morbidities with major depression (Davis et al, 2008). Our analysis without these subjects demonstrated a similar result (reduced FA in the right ALIC) albeit non-significant. Given p value of 0.078, it is likely that the lack of significance was due to the sample size reduction rather than an inherent effect of a history of substance abuse. Furthermore, removing these subjects did not significantly change the effect size with Cohen’s d reduced from 0.48 to 0.33 (z=0.26, P=0.4). This suggests that a history of alcohol and/or marijuana abuse in our depressed sample did not have a significant impact on measures of white matter integrity. In addition, the pattern of significant correlations without these subjects remained the same. We also note that the use of the CES-D, a self-rated scale, as a measure of depression severity may be a limitation. However, we used it since HAM-D scores were used in the determination of subject eligibility. Our results must also be interpreted in the context of medication factors which we did not control for. Even though all depressed subjects were antidepressant-free, other medications, such as oral hypoglyemics, may have altered extracellular fluid compartment water, especially in diabetic subjects, thus impacting FA measurements.
One important limitation of this study is in the reproducibility of the extracted ALIC tracts. However, although our one-ROI approach may seem to imply substantial compromise in the tract reproducibility, we demonstrated reasonable inter- and intra-rater reliabilities. Additionally, as noted in the Methods, the reconstructed fibers as reported here underwent several careful rounds of virtual dissection in order to remove fibers not consistent with the tracts in the ALIC. As the virtual dissection was carefully conducted (after initial candidate fibers were generated using the said first ROI), a substantial percentage of our final reconstructed fibers were nevertheless extracted by selecting additional ROIs, applied in concert with the first ROI by a logical “AND”, thus allowing us to greatly minimize potential false-positive erroneous fibers. There may also be additional limitations in our MRI protocol and image acquisition. With regard to the DTI acquisition, we applied the well-established protocol optimized for Philips 3T scanners, with a SENSE factor of 2.5 chosen to well balance the scan time and the signal-to-noise ratio (Reich et al, 2006). Note that the sequence uses a lower b-value of 700 s/mm2 to reduce eddy current effects. However, any residual effects were further minimized by the coregistation step in image post-processing before tensor fitting. Although we did not perform cardiac gating due to time constraints, we carefully conducted quality control on the raw images and removed all subjects with artifacts due to head movement. Lastly, our ratio of non-diffusion weighted to diffusion weighted volumes may not be optimal based on the criteria proposed in (Smith et al, 2007), which recommends 1 non-diffusion weighted for every 8 diffusion weighted images.
In summary, based on previous findings suggesting the role of select regions of the frontal cortex and striatal region in mood regulation in major depression and diabetes, we investigated the white matter microstructural integrity of the ALIC in depression and diabetes using DTI-tractography. Our study makes the following novel contributions in clarifying tract-specific abnormalities in major depression: 1) supports the involvement of the ALIC in the pathology of MDD; 2) demonstrates that radial diffusivity has a trend towards significant alteration in the right ALIC; and 3) links hgb A1c in diabetic subjects with FA in the left ALIC. This study complements the growing literature on the neuroanatomical correlates of the pathophysiology of depression and diabetes. Future work needs to be done to understand the longitudinal effects of these structural connectivity alterations and their functional consequences.
References
Ajilore O, Haroon E, Kumaran S, Darwin C, Binesh N, Mintz J et al (2007). Measurement of brain metabolites in patients with type 2 diabetes and major depression using proton magnetic resonance spectroscopy. Neuropsychopharmacology 32: 1224–1231.
Alexander GE, Crutcher MD, DeLong MR (1990). Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, "prefrontal" and "limbic" functions. Prog Brain Res 85: 119–146.
Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Latoussakis V, Kanellopoulos D, Klimstra S et al (2008). Microstructural white matter abnormalities and remission of geriatric depression. Am J Psychiatry 165: 238–244.
Anderson RJ, Freedland KE, Clouse RE, Lustman PJ (2001). The prevalence of comorbid conditions in adults with diabetes: a meta-analysis. Diabetes Care 24: 1069–1078.
Axer H, Keyserlingk DG (2000). Mapping of fiber orientation in human internal capsule by means of polarized light and confocal scanning laser microscopy. J Neurosci Meth 94: 165–175.
Bae JN, MacFall JR, Krishnan KR, Payne ME, Steffens DC, Taylor WD (2006). Dorsolateral prefrontal cortex and anterior cingulate cortex white matter alterations in late-life depression. Biol Psychiatry 60: 1356–1363.
Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N et al (2010). Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage 53: 1244–1255.
Bartzokis G (2012). Neuroglialpharmacology: Myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology 62: 2136–2152.
Bora E, Harrison BJ, Davey CG, Yücel M, Pantelis C (2011). Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in majordepressive disorder. Psychol Med 1–11.
Brassington JC, Marsh NV (1998). Neuropsychological aspects of multiple sclerosis. Neuropsychol Rev 8: 43–77.
Carpenter MB (1991) Core text of neuroanatomy 4th ed. Williams & Wilkins: Baltimore.
Ciccarelli O, Werring DJ, Wheeler-Kingshott CAM, Barker GJ, Parker GJM, Thompson AJ et al (2001). Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology 56: 926–933.
Cunningham DJ (1903). Text-book of Anatomy. Pentland.
Davis L, Uezato A, Newell JM, Frazier E (2008). Major depression and comorbid substance use disorders. Curr Opin Psychiatry 21: 14–18.
First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS (1997) Structured Clinical Interview for DSM-IV Axis I Disorders-Clinical Version (SCID-CV).. American Psychiatric Publishing: Washington, DC.
Gutman DA, Holtzheimer PE, Behrens TE, Johansen-Berg H, Mayberg HS (2009). A tractography analysis of two deep brain stimulation white matter targets for depression. Biol Psychiatry 65: 276–282.
Hamilton M (1967). Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296.
Hsu JL, Chen YL, Leu JG, Jaw FS, Lee CH, Tsai YF et al (2012). Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage 59: 1098–1105.
Jia Z, Huang X, Wu Q, Zhang T, Lui S, Zhang J et al (2010). High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry 167: 1381–1390.
Jiang H, van Zijl PC, Kim J, Pearlson GD, Mori S (2006). DtiStudio: Resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Programs Biomed 81: 106–116.
Kahle W, Platzer W, Frotscher M, Leonhardt H (2002) Color Atlas and Textbook of Human Anatomy. Thieme, Medical Publishers, Inc: New York.
Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S et al (2011). Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Arch Gen Psychiatry 68: 675–690.
Krishnan MS, O'Brien JT, Firbank MJ, Pantoni L, Carlucci G, Erkinjuntti T et alLADIS Group (2006). Relationship between periventricular and deep white matter lesions and depressive symptoms in older people. The LADIS Study. Int J Geriatr Psychiatry 21: 983–989.
Kumar A, Cook IA (2002). White matter injury, neural connectivity and the pathophysiology of psychiatric disorders. Dev Neurosci 24: 255–261.
Kumar A, Gupta R, Thomas A, Ajilore O, Hellemann G (2009). Focal Subcortical Biophysical Abnormalities in Patients Diagnosed With Type 2 Diabetes and Depression. Arch Gen Psychiatry 66: 324–330.
Leonard E, Zheng D, Simpson K (2002). Comorbid Depression is Associated With Increased Health Care Use and Expenditures in Individuals With Diabetes. Diabetes Care 25: 464–470.
Machado A, Haber S, Sears N, Greenberg B, Malone D, Rezai A (2009). Functional topography of the ventral striatum and anterior limb of the internal capsule determined by electrical stimulation of awake patients. Clinical Neurophysiology 120: 1941–1948.
Mettenburg JM, Benzinger TL, Shimony JS, Snyder AZ, Sheline YI (2012). Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage 60: 2182–2190.
Mori S, van Zijl PC (2002). Fiber tracking: principles and strategies – a technical review. NMR Biomed 15: 468–480.
Nichols GA, Brown JB (2003). Unadjusted and adjusted prevalence of diagnosed depression in type 2 diabetes. Diabetes Care 26: 744–749.
Phillips JL, Batten LA, Aldosary F, Tremblay P, Blier P (2012). Brain-volume increase with sustained remission in patients with treatment-resistant unipolar depression. J Clin Psychiatry 73: 625–631.
Radloff LS (1977). The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1: 385–401.
Rustad JK, Musselman DL, Nemeroff CB (2011). The relationship of depression and diabetes: pathophysiological and treatment implications. Psychoneuroendocrinology 36: 1276–1286.
Shimony JS, Sheline YI, D'Angelo G, Epstein AA, Benzinger TL, Mintun MA et al (2009). Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol Psychiatry 66: 245–252.
Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL et al (2007). Acquisition and voxelwise analysis of multi-subject diffusion data with Tract-Based Spatial Statistics. Nature Protocols 2: 499–503.
Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH (2003). Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 20: 1714–1722.
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17: 1429–1436.
Taylor WD, Payne ME, Krishnan KR, Wagner HR, Provenzale JM, Steffens DC et al (2001). Evidence of white matter tract disruption in MRI hyperintensities. Biol Psychiatry 50: 179–183.
Tillema J, Leach J, Pirko I (2012). Non-lesional white matter changes in pediatric multiple sclerosis and monophasic demyelinating disorders. Mult Scler 18: 1754–1759.
Williams VJ, Leritz EC, Shepel J, McGlinchey RE, Milberg WP, Rudolph JL et al (2012). Interindividual variation in serum cholesterol is associated with regional white matter tissue integrity in older adults. Hum Brain Mapp (e-pub ahead of print).
Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC (1998). Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography 22: 139–152.
Woods RP, Grafton ST, Watson JDG, Sicotte NL, Mazziotta JC (1998). Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography 22: 153–165.
Zhang A, Leow A, Ajilore O, Lamar M, Yang S, Joseph J et al (2012). Quantitative Tract-Specific Measures of Uncinate and Cingulum in Major Depression Using Diffusion Tensor Imaging. Neuropsychopharmacology 37: 959–967.
Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S (2011). Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res 1369: 223–229.
Zou K, Huang X, Li T, Gong Q, Li Z, Ou-yang L et al (2008). Alterations of white matter integrity in adults with major depressive disorder: a magnetic resonance imaging study. J Psychiatry Neurosci 33: 525–530.
Acknowledgements
This research was supported by NIH grants 5R01MH63764-7, 5R01MH73989-5 and 5K23MH081175-02, also by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We would like to thank Ms. Monya Meinel, Ms. Mai Lynn Grajewski and Mr. Piotr Daranowski for their assistance in coordinating this study and Dr. Dulal Bhamik for his helpful comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The paper was previously presented at the Society for Neuroscience 2012 Annual Meeting in New Orleans, Louisiana, USA.
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, A., Ajilore, O., Zhan, L. et al. White Matter Tract Integrity of Anterior Limb of Internal Capsule in Major Depression and Type 2 Diabetes. Neuropsychopharmacol 38, 1451–1459 (2013). https://doi.org/10.1038/npp.2013.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.41
Keywords
This article is cited by
-
Psychopathological network for early-onset post-stroke depression symptoms
BMC Psychiatry (2023)
-
Microstructural brain tissue changes contribute to cognitive and mood deficits in adults with type 2 diabetes mellitus
Scientific Reports (2023)
-
Brain conditions mediate the association between aging and happiness
Scientific Reports (2022)
-
Oligodendrocyte lineage cells and depression
Molecular Psychiatry (2021)
-
Correlation between white matter microstructure changes and cognitive function in type 2 diabetes assessed using diffusion tensor imaging
Chinese Journal of Academic Radiology (2021)