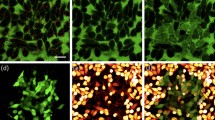
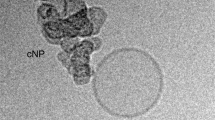
Abstract
A major barrier to drug and gene delivery is crossing the cell's plasma membrane. Physical forces applied to cells via electroporation1, ultrasound2 and laser irradiation3,4,5,6 generate nanoscale holes in the plasma membrane for direct delivery of drugs into the cytoplasm. Inspired by previous work showing that laser excitation of carbon nanoparticles can drive the carbon-steam reaction to generate highly controlled shock waves7,8,9,10, we show that carbon black nanoparticles activated by femtosecond laser pulses can facilitate the delivery of small molecules, proteins and DNA into two types of cells. Our initial results suggest that interaction between the laser energy and carbon black nanoparticles may generate photoacoustic forces by chemical reaction to create transient holes in the membrane for intracellular delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bodles-Brakhop, A. M., Heller, R. & Draghia-Akli, R. Electroporation for the delivery of DNA-based vaccines and immunotherapeutics: current clinical developments. Mol. Ther. 17, 585–592 (2009).
Mitragotri, S. Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nature Rev. Drug Discov. 4, 255–260 (2005).
Tao, W., Wilkinson, J., Stanbridge, E. J. & Berns, M. W. Direct gene transfer into human cultured cells facilitated by laser micropuncture of the cell membrane. Proc. Natl Acad. Sci. USA 84, 4180–4184 (1987).
Tirlapur, U. K. & Konig, K. Targeted transfection by femtosecond laser. Nature 418, 290–291 (2002).
Kodama, T., Doukas, A. G. & Hamblin, M. R. Shock wave-mediated molecular delivery into cells. Biochim. Biophys. Acta 1542, 186–194 (2002).
Esenaliev, R. O., Larina, I. V., Larin, K. V., Motamedi, M. & Evers, B. M. Mechanism of laser-induced drug delivery in tumors. Proc. SPIE 3914, 188–196 (2000).
Löwen, H. & Madden, P. A. A microscopic mechanism for shock-wave generation in pulsed-laser-heated colloidal suspensions. J. Chem. Phys. 97, 8760–8766 (1992).
Chen, H. & Diebold, G. Chemical generation of acoustic waves: a giant photoacoustic effect. Science 270, 963–966 (1995).
Chen, H., McGrath, T. & Diebold, G. J. Laser chemistry in suspensions: new products and unique reaction conditions for the carbon-steam reaction. Angew. Chem. Int. Ed. Engl. 36, 163–166 (1997).
McGrath, T. E., Diebold, G. J., Bartels, D. M. & Crowell, R. A. Laser-initiated chemical reactions in carbon suspensions. J. Phys. Chem. A 106, 10072–10078 (2002).
Lu, S., Wang, S. & Grimes-Serrano, J. M. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines 7, 175–191 (2008).
Whitehead, K. A., Langer, R. & Anderson, D. G. Knocking down barriers: advances in siRNA delivery. Nature Rev. Drug Discov. 8, 129–138 (2009).
Desnick, R. J. Enzyme replacement and enhancement therapies for lysosomal diseases. J. Inherit. Metab. Dis. 27, 385–410 (2004).
Nobili, S., Landini, I., Giglioni, B. & Mini, E. Pharmacological strategies for overcoming multidrug resistance. Curr. Drug Targets 7, 861–879 (2006).
Young, L. S., Searle, P. F., Onion, D. & Mautner, V. Viral gene therapy strategies: from basic science to clinical application. J. Pathol. 208, 299–318 (2006).
Lavigne, M. D. & Gorecki, D. C. Emerging vectors and targeting methods for nonviral gene therapy. Expert Opin. Emerg. Drugs 11, 541–557 (2006).
Mehier-Humbert, S. & Guy, R. H. Physical methods for gene transfer: improving the kinetics of gene delivery into cells. Adv. Drug Deliv. Rev. 57, 733–753 (2005).
Esenaliev, R. O. Application of light and ultrasound for medical diagnostics and treatment. Proc. SPIE 4707, 158–164 (2002).
ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 93, 111–129 (2007).
Hernot, S. & Klibanov, A. L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 60, 1153–1166 (2008).
Hynynen, K. Ultrasound for drug and gene delivery to the brain. Adv. Drug Deliv. Rev. 60, 1209–1217 (2008).
Lukacs, G. L. et al. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 275, 1625–1629 (2000).
Sun, X. et al. Investigation of an optical limiting mechanism in multiwalled carbon manotubes. Appl. Opt. 39, 1998–2001 (2000).
Link, S., Burda, C., Nikoobakht, B. & El-Sayed, M. A. Laser-induced shape changes of colloidal gold nanorods using femtosecond and nanosecond laser pulses. J. Phys. Chem. B 104, 6152–6163 (2000).
Fayer, M. Picosecond holographic grating generation of ultrasonic waves. IEEE J. Quant. Electron. 22, 1437–1452 (1986).
Brukh, R. & Mitra, S. Kinetics of carbon nanotube oxidation. J. Mater. Chem. 17, 619–623 (2007).
Zhao, N., He, C., Jiang, Z., Li, J. & Li, Y. Physical activation and characterization of multi-walled carbon nanotubes catalytically synthesized from methane. Mater. Lett. 61, 681–685 (2007).
Huang, X., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
Zarnitsyn, V. G. & Prausnitz, M. R. Physical parameters influencing optimization of ultrasound-mediated DNA transfection. Ultrasound Med. Biol. 30, 527–538 (2004).
Guzman, H. R., Nguyen, D. X., Khan, S. & Prausnitz, M. R. Ultrasound-mediated disruption of cell membranes I: quantification of molecular uptake and cell viability. J. Acoust. Soc. Am. 110, 588–596 (2001).
Schlicher, R. K. et al. Mechanism of intracellular delivery by acoustic cavitation. Ultrasound Med. Biol. 32, 915–924 (2006).
Acknowledgements
The authors thank S. Graham for use of his pressure measurement apparatus, S. Nair for technical discussions, and J.-H. Park, D. Hallow, R. Schlicher, J. Hutcheson and Y. Liu for helpful discussions and laboratory assistance. This work was supported in part by the U.S. National Institutes of Health and Institute of Paper Science and Technology and was carried out in the Institute for Bioengineering and Biosciences, Center for Drug Design, Development and Delivery, and Laser Dynamics Laboratory at Georgia Tech.
Author information
Authors and Affiliations
Contributions
P.C. carried out the study in collaboration with W.Q., who provided laser expertise. M.R.P. and M.A.E. were the principal investigators. All authors participated in study design and interpretation. P.C. and M.R.P. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 601 kb)
Rights and permissions
About this article
Cite this article
Chakravarty, P., Qian, W., El-Sayed, M. et al. Delivery of molecules into cells using carbon nanoparticles activated by femtosecond laser pulses. Nature Nanotech 5, 607–611 (2010). https://doi.org/10.1038/nnano.2010.126
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.126
This article is cited by
-
Microsecond cell triple-sorting enabled by multiple pulse irradiation of femtosecond laser
Scientific Reports (2023)
-
Advances in Ultra-small Fluorescence Nanoprobes for Detection of Metal Ions, Drugs, Pesticides and Biomarkers
Journal of Fluorescence (2023)
-
Laser-based molecular delivery and its applications in plant science
Plant Methods (2022)
-
Light triggered nanoscale biolistics for efficient intracellular delivery of functional macromolecules in mammalian cells
Nature Communications (2022)
-
Dynamic blood flow phantom for in vivo liquid biopsy standardization
Scientific Reports (2021)