Abstract
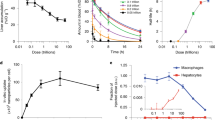
Inorganic/organic hybrid nanoparticles are potentially useful in biomedicine, but to avoid non-specific background fluorescence and long-term toxicity, they need to be cleared from the body within a reasonable timescale1. Previously, we have shown that rigid spherical nanoparticles such as quantum dots can be cleared by the kidneys if they have a hydrodynamic diameter of approximately 5.5 nm and a zwitterionic surface charge2. Here, we show that quantum dots functionalized with high-affinity small-molecule ligands that target tumours can also be cleared by the kidneys if their hydrodynamic diameter is less than this value, which sets an upper limit of 5–10 ligands per quantum dot for renal clearance. Animal models of prostate cancer and melanoma show receptor-specific imaging and renal clearance within 4 h post-injection. This study suggests a set of design rules for the clinical translation of targeted nanoparticles that can be eliminated through the kidneys.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Frangioni, J. V. New technologies for human cancer imaging. J. Clin. Oncol. 26, 4012–4021 (2008).
Choi, H. S. et al. Renal clearance of quantum dots. Nature Biotechnol. 25, 1165–1170 (2007).
Alivisatos, P. The use of nanocrystals in biological detection. Nature Biotechnol. 22, 47–52 (2004).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 22, 969–976 (2004).
Michalet, X. et al. Quantum dots for live cells, in vivo imaging and diagnostics. Science 307, 538–544 (2005).
Medintz, I. L., Uyeda, H. T., Goldman, E. R. & Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nature Mater. 4, 435–446 (2005).
Harisinghani, M. G. et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 348, 2491–2499 (2003).
Frangioni, J. V. Translating in vivo diagnostics into clinical reality. Nature Biotechnol. 24, 909–913 (2006).
Colvin, V. The potential environmental impact of engineered nanomaterials. Nature Biotechnol. 21, 1166–1170 (2003).
Duncan, R. Polymer conjugates as anticancer nanomedicines. Nature Rev. Cancer 6, 688–701 (2006).
Weissleder, R., Kelly, K., Sun, E. Y., Shtatland, T. & Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature Biotechnol. 23, 1418–1423 (2005).
Ballou, B. et al. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug. Chem. 18, 389–396 (2007).
Qian, X. et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnol. 26, 83–90 (2008).
Peer, D. et al. Nanocarriers: emerging platforms for cancer therapy. Nature Nanotech. 2, 751–760 (2007).
Hamad, I., Hunter, A. C., Szebeni, J. & Moghimi, S. M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 46, 225–232 (2008).
Dobrovolskaia, M. A. & McNeil, S. E. Immunological properties of engineered nanomaterials. Nature Nanotech. 2, 469–478 (2007).
Yang, J. et al. Development of aliphatic biodegradable photoluminescent polymers. Proc. Natl Acad. Sci. USA 106, 10086–10091 (2009).
Barstow, L. & Small, R. E. Liver function assessment by drug metabolism. Pharmacotherapy 10, 280–288 (1990).
McAfee, J. G. et al. Technetium-99 m DADS complexes as renal function and imaging agents: II. Biological comparison with iodine-131 hippuran. J. Nucl. Med. 26, 375–384 (1985).
Cai, W. et al. Peptide-labeled near-infrared quantum dots for imaging tumor vasculature in living subjects. Nano Lett. 6, 669–676 (2006).
Smith, A. M., Duan, H., Mohs, A. M. & Nie, S. Bioconjugated quantum dots for in vivo molecular and cellular imaging. Adv. Drug Deliv. Rev. 60, 1226–1240 (2008).
Chen, K., Li, Z. B., Wang, H., Cai, W. & Chen, X. Dual-modality optical and positron emission tomography imaging of vascular endothelial growth factor receptor on tumor vasculature using quantum dots. Eur. J. Nucl. Med. Mol. Imaging 35, 2235–2244 (2008).
Bentolila, L. A., Ebenstein, Y. & Weiss, S. Quantum dots for in vivo small-animal imaging. J. Nucl. Med. 50, 493–496 (2009).
Matsumura, Y., Oda, T. & Maeda, H. General mechanism of intratumor accumulation of macromolecules: advantage of macromolecular therapeutics. Gan To Kagaku Ryoho 14, 821–829 (1987).
Humblet, V. et al. High-affinity near-infrared fluorescent small-molecule contrast agents for in vivo imaging of prostate-specific membrane antigen. Mol. Imaging 4, 448–462 (2005).
Chen, X., Park, R., Shahinian, A. H., Bading, J. R. & Conti, P. S. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl. Med. Biol. 31, 11–19 (2004).
Liu, W. et al. Compact cysteine-coated CdSe(ZnCdS) quantum dots for in vivo applications. J. Am. Chem. Soc. 129, 14530–14531 (2007).
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev. Drug Discov. 7, 771–782 (2008).
Choi, H. S. et al. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 9, 2354–2359 (2009).
Tanaka, E., Choi, H. S., Fujii, H., Bawendi, M. G. & Frangioni, J. V. Image-guided oncologic surgery using invisible light: completed pre-clinical development for sentinel lymph node mapping. Ann. Surg. Oncol. 13, 1671–1681 (2006).
Acknowledgements
Support from the Biophysical Instrumentation Facility for the Study of Complex Macromolecular Systems (NSF-0070319 and NIH GM68762) is gratefully acknowledged. This work was supported in part by NIH grant no. R33-EB-000673 (J.V.F. and M.G.B), NIH grant no. R01-CA-115296 (J.V.F.), and a fellowship from the Charles A. King Trust, Bank of America, Co-Trustee (H.S.C.). M.G.B. also acknowledges support from the NIH-funded MIT-Harvard NanoMedical Consortium (1U54-CA119349, a Center of Cancer Nanotechnology Excellence).
Author information
Authors and Affiliations
Contributions
H.S.C., W.L., F.L., K.N. and P.M. performed the experiments. H.S.C., M.G.B. and J.V.F. reviewed, analysed and interpreted the data. H.S.C., M.G.B. and J.V.F. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 1436 kb)
Rights and permissions
About this article
Cite this article
Choi, H., Liu, W., Liu, F. et al. Design considerations for tumour-targeted nanoparticles. Nature Nanotech 5, 42–47 (2010). https://doi.org/10.1038/nnano.2009.314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2009.314