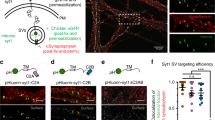
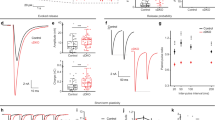
Abstract
Presynaptic inhibition mediated by G protein–coupled receptors may involve a direct interaction between G proteins and the vesicle fusion machinery. The molecular target of this pathway is unknown. We demonstrate that Gβγ-mediated presynaptic inhibition in lamprey central synapses occurs downstream from voltage-gated Ca2+ channels. Using presynaptic microinjections of botulinum toxins (BoNTs) during paired recordings, we find that cleavage of synaptobrevin in unprimed vesicles leads to an eventual exhaustion of synaptic transmission but does not prevent Gβγ-mediated inhibition. In contrast, cleavage of the C-terminal nine amino acids of the 25 kDa synaptosome-associated protein (SNAP-25) by BoNT A prevents Gβγ-mediated inhibition. Moreover, a peptide containing the region of SNAP-25 cleaved by BoNT A blocks the Gβγ inhibitory effect. Finally, removal of the last nine amino acids of the C-terminus of SNAP-25 weakens Gβγ interactions with soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complexes. Thus, the C terminus of SNAP-25, which links synaptotagmin I to the SNARE complex, may represent a target of Gβγ for presynaptic inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Holz, G.G., 4th., Kream, R.M., Spiegel, A. & Dunlap, K. G proteins couple α-adrenergic and GABAb receptors to inhibition of peptide secretion from peripheral sensory neurons. J. Neurosci. 9, 657–666 (1989).
Dolphin, A.C. et al. G protein modulation of voltage-dependent calcium channels and transmitter release. Biochem. Soc. Trans. 21, 391–395 (1993).
De Waard, M. et al. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature 385, 446–450 (1997).
Qin, N., Platano, D., Olcese, R., Stefani, E. & Birnbaumer, L. Direct interaction of gβγ with a C-terminal gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc. Natl. Acad. Sci. USA 94, 8866–8871 (1997).
Kajikawa, Y., Saitoh, N. & Takahashi, T. GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABA(B) receptor. Proc. Natl. Acad. Sci. USA 98, 8054–8058 (2001).
Hamm, H.E. The many faces of G protein signaling. J. Biol. Chem. 273, 669–672 (1998).
Blackmer, T. et al. G protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science 292, 293–297 (2001).
Takahashi, M., Freed, R., Blackmer, T. & Alford, S. Calcium influx-independent depression of transmitter release by 5-HT at lamprey spinal cord synapses. J. Physiol. (Lond.) 532, 323–336 (2001).
Silinsky, E.M. & Solsona, C.S. Calcium currents at motor nerve endings: absence of effects of adenosine receptor agonists in the frog. J. Physiol. (Lond.) 457, 315–328 (1992).
Jarvis, S.E., Magga, J.M., Beedle, A.M., Braun, J.E. & Zamponi, G.W. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. J. Biol. Chem. 275, 6388–6394 (2000).
Blackmer, T. et al. G protein βγ directly regulates the SNARE protein fusion machinery for secretory granule exocytosis. Nat. Neurosci. 8, 421–425 (2005).
Brodin, L., Buchanan, J.T., Hokfelt, T., Grillner, S. & Verhofstad, A.A. A spinal projection of 5-hydroxytryptamine neurons in the lamprey brainstem; evidence from combined retrograde tracing and immunohistochemistry. Neurosci. Lett. 67, 53–57 (1986).
Brodin, L., Shupliakov, O. & Grillner, S.E. Central glutamatergic transmission. A view from the presynaptic axon. Adv. Second Messenger Phosphoprotein Res. 29, 205–221 (1994).
Brodin, L. et al. Reticulospinal neurons in lamprey: transmitters, synaptic interactions and their role during locomotion. Arch. Ital. Biol. 126, 317–345 (1988).
Gustafsson, J.S. et al. Ultrastructural organization of lamprey reticulospinal synapses in three dimensions. J. Comp. Neurol. 450, 167–182 (2002).
Shupliakov, O., Pieribone, V.A., Gad, H. & Brodin, L. Synaptic vesicle depletion in reticulospinal axons is reduced by 5-hydroxytryptamine: direct evidence for presynaptic modulation of glutamatergic transmission. Eur. J. Neurosci. 7, 1111–1116 (1995).
Neher, E. & Zucker, R.S. Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron 10, 21–30 (1993).
Delaney, K.R. & Zucker, R.S. Calcium released by photolysis of DM-nitrophen stimulates transmitter release at squid giant synapse. J. Physiol. (Lond.) 426, 473–498 (1990).
Mulkey, R.M. & Zucker, R.S. Calcium released by photolysis of DM-nitrophen triggers transmitter release at the crayfish neuromuscular junction. J. Physiol. (Lond.) 462, 243–260 (1993).
Niemann, H., Blasi, J. & Jahn, R. Clostridial neurotoxins: new tools for dissecting exocytosis. Trends Cell Biol. 4, 179–185 (1994).
Montecucco, C. & Schiavo, G. Structure and function of tetanus and botulinum neurotoxins. Q. Rev. Biophys. 28, 423–472 (1995).
Williamson, L.C., Halpern, J.L., Montecucco, C., Brown, J.E. & Neale, E.A. Clostridial neurotoxins and substrate proteolysis in intact neurons: botulinum neurotoxin C acts on synaptosomal-associated protein of 25 kDa. J. Biol. Chem. 271, 7694–7699 (1996).
Schiavo, G., Matteoli, M. & Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 80, 717–766 (2000).
Humeau, Y., Doussau, F., Grant, N.J. & Poulain, B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 82, 427–446 (2000).
Ahnert-Hilger, G. & Bigalke, H. Molecular aspects of tetanus and botulinum neurotoxin poisoning. Prog. Neurobiol. 46, 83–96 (1995).
Banerjee, A., Kowalchyk, J.A., DasGupta, B.R. & Martin, T.F. SNAP-25 is required for a late postdocking step in Ca2+-dependent exocytosis. J. Biol. Chem. 271, 20227–20230 (1996).
Schiavo, G. et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359, 832–835 (1992).
Hanson, M.A. & Stevens, R.C. Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 A resolution. Nat. Struct. Biol. 7, 687–692 (2000).
Hayashi, T. et al. Synaptic vesicle membrane fusion complex: action of clostridial neurotoxins on assembly. EMBO J. 13, 5051–5061 (1994).
Pellegrini, L.L., O'Connor, V. & Betz, H. Fusion complex formation protects synaptobrevin against proteolysis by tetanus toxin light chain. FEBS Lett. 353, 319–323 (1994).
Magga, J.M., Jarvis, S.E., Arnot, M.I., Zamponi, G.W. & Braun, J.E. Cysteine string protein regulates G protein modulation of N-type calcium channels. Neuron 28, 195–204 (2000).
Schiavo, G. et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 335, 99–103 (1993).
Binz, T. et al. Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J. Biol. Chem. 269, 1617–1620 (1994).
Otto, H., Hanson, P.I., Chapman, E.R., Blasi, J. & Jahn, R. Poisoning by botulinum neurotoxin A does not inhibit formation or disassembly of the synaptosomal fusion complex. Biochem. Biophys. Res. Commun. 212, 945–952 (1995).
Xu, T., Binz, T., Niemann, H. & Neher, E. Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nat. Neurosci. 1, 192–200 (1998).
Wei, S. et al. Exocytotic mechanism studied by truncated and zero layer mutants of the C-terminus of SNAP-25. EMBO J. 19, 1279–1289 (2000).
Tucker, W.C., Weber, T. & Chapman, E.R. Reconstitution of Ca2+-regulated membrane fusion by synaptotagmin and SNAREs. Science 304, 435–438 (2004).
Zhang, X., Kim-Miller, M.J., Fukuda, M., Kowalchyk, J.A. & Martin, T.F. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron 34, 599–611 (2002).
Gerona, R.R., Larsen, E.C., Kowalchyk, J.A. & Martin, T.F. The C terminus of SNAP25 is essential for Ca2+-dependent binding of synaptotagmin to SNARE complexes. J. Biol. Chem. 275, 6328–6336 (2000).
Pinxteren, J.A., O'Sullivan, A.J., Tatham, P.E. & Gomperts, B.D. Regulation of exocytosis from rat peritoneal mast cells by G protein βγ-subunits. EMBO J. 17, 6210–6218 (1998).
Lang, J. Molecular mechanisms and regulation of insulin exocytosis as a paradigm of endocrine secretion. Eur. J. Biochem. 259, 3–17 (1999).
Blasi, J. et al. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365, 160–163 (1993).
Bruns, D. et al. Inhibition of transmitter release correlates with the proteolytic activity of tetanus toxin and botulinus toxin A in individual cultured synapses of Hirudo medicinalis. J. Neurosci. 17, 1898–1910 (1997).
Schmidt, J.J. & Bostian, K.A. Endoproteinase activity of type A botulinum neurotoxin: substrate requirements and activation by serum albumin. J. Protein Chem. 16, 19–26 (1997).
Fernandez-Chacon, R. et al. The synaptic vesicle protein CSP α prevents presynaptic degeneration. Neuron 42, 237–251 (2004).
Schwartz, E.J., Gerachshenko, T. & Alford, S. 5-HT prolongs ventral root bursting via presynaptic inhibition of synaptic activity during fictive locomotion in lamprey. J. Neurophysiol. 93, 980–988 (2005).
Ford, C.E. et al. Molecular basis for interactions of G protein βγ subunits with effectors. Science 280, 1271–1274 (1998).
Blanes-Mira, C. et al. Identification of SNARE complex modulators that inhibit exocytosis from an α-helix-constrained combinatorial library. Biochem. J. 375, 159–166 (2003).
Phillips, W.J. & Cerione, R.A. Labeling of the βγ subunit complex of transducin with an environmentally sensitive cysteine reagent. Use of fluorescence spectroscopy to monitor transducin subunit interactions. J. Biol. Chem. 266, 11017–11024 (1991).
Sutton, R.B., Fasshauer, D., Jahn, R. & Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395, 347–353 (1998).
Acknowledgements
We would like to thank T.F.J. Martin, J. Richmond, E. Schwartz, H. Photowala, R. Smetana and S. Ramakrishna for critical reading of the manuscript and/or discussions. Thanks to C. Malizio (Metabiologics, Inc.) for special preparation of BoNT A. The work was supported by grants from the National Institutes of Neurological Disorders and Stroke, The National Institute of Mental Health and the National Science Foundation to S.A. and the National Eye Institute to H.E.H.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
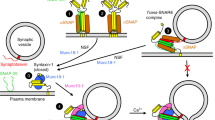
Supplementary Fig. 1
Model of 5-HT GPCR signaling depicts presynaptic and postsynaptic sites of the lamprey giant synapse. The 5-HT receptor signals through free Gβγ. Gβγ can access assembled SNARE complexes to inhibit synaptic transmission. Presynaptically applied BoNT/A and ct-SNAP-25 block this inhibition suggesting that Gβγ acts at the C-terminus of SNAP-25. (PDF 778 kb)
Rights and permissions
About this article
Cite this article
Gerachshenko, T., Blackmer, T., Yoon, EJ. et al. Gβγ acts at the C terminus of SNAP-25 to mediate presynaptic inhibition. Nat Neurosci 8, 597–605 (2005). https://doi.org/10.1038/nn1439
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1439
This article is cited by
-
Postsynaptic synucleins mediate endocannabinoid signaling
Nature Neuroscience (2023)
-
Physiological roles for neuromodulation via Gi/o GPCRs working through Gβγ–SNARE interaction
Neuropsychopharmacology (2020)
-
Molecular pharmacology of metabotropic receptors targeted by neuropsychiatric drugs
Nature Structural & Molecular Biology (2019)
-
Gβγ SNARE Interactions and Their Behavioral Effects
Neurochemical Research (2019)
-
SNAP-25b-deficiency increases insulin secretion and changes spatiotemporal profile of Ca2+oscillations in β cell networks
Scientific Reports (2017)