Abstract
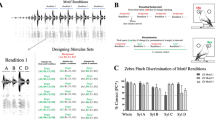
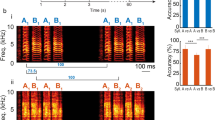
The division of continuously variable acoustic signals into discrete perceptual categories is a fundamental feature of vocal communication, including human speech. Despite the importance of categorical perception to learned vocal communication, the neural correlates underlying this phenomenon await identification. We found that individual sensorimotor neurons in freely behaving swamp sparrows expressed categorical auditory responses to changes in note duration, a learned feature of their songs, and that the neural response boundary accurately predicted the categorical perceptual boundary measured in field studies of the same sparrow population. Furthermore, swamp sparrow populations that learned different song dialects showed different categorical perceptual boundaries that were consistent with the boundary being learned. Our results extend the analysis of the neural basis of perceptual categorization into the realm of vocal communication and advance the learned vocalizations of songbirds as a model for investigating how experience shapes categorical perception and the activity of categorically responsive neurons.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Miller, E.K., Nieder, A., Freedman, D.J. & Wallis, J.D. Neural correlates of categories and concepts. Curr. Opin. Neurobiol. 13, 198–203 (2003).
Bregman, A. Auditory Scene Analysis (MIT Press, Cambridge, Massachusetts, 1990).
Liberman, A.M., Harris, K.S., Hoffman, H.S. & Griffith, B.C. The discrimination of speech sounds within and across phoneme boundaries. J. Exp. Psychol. 54, 358–368 (1957).
Liberman, A.M., Harris, K.S., Kinney, J.A. & Lane, H. The discrimination of relative onset-time of the components of certain speech and nonspeech patterns. J. Exp. Psychol. 61, 379–388 (1961).
May, B., Moody, D.B. & Stebbins, W.C. Categorical perception of conspecific communication sounds by Japanese macaques, Macaca fuscata. J. Acoust. Soc. Am. 85, 837–847 (1989).
Kuhl, P.K. & Miller, J.D. Speech-perception by chinchilla: phonetic boundaries for synthetic VOT stimuli. J. Acoust. Soc. Am. 57, S49–S50 (1975).
Nelson, D.A. & Marler, P. Categorical perception of a natural stimulus continuum: birdsong. Science 244, 976–978 (1989).
Baugh, A.T., Akre, K.L. & Ryan, M.J. Categorical perception of a natural, multivariate signal: mating call recognition in tungara frogs. Proc. Natl. Acad. Sci. USA 105, 8985–8988 (2008).
Nowak, M.A. & Krakauer, D.C. The evolution of language. Proc. Natl. Acad. Sci. USA 96, 8028–8033 (1999).
Marler, P. & Peters, S. Sparrows learn adult song and more from memory. Science 213, 780–782 (1981).
Catchpole, C.K. & Slater, P.J.B. Birdsong: Biological Themes and Variations (Cambridge University Press, New York, 1995).
Marler, P. & Pickert, R. Species-universal microstructure in the learned song of the swamp sparrow (Melospiza georgiana). Anim. Behav. 32, 673–689 (1984).
Clark, C.W., Marler, P. & Beeman, K. Quantitative analysis of animal vocal phonology: an application to swamp sparrow song. Ethology 76, 101–115 (1987).
Nottebohm, F., Stokes, T.M. & Leonard, C.M. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457–486 (1976).
Gentner, T.Q., Hulse, S.H., Bentley, G.E. & Ball, G.F. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning and categorization of conspecific song in adult songbirds. J. Neurobiol. 42, 117–133 (2000).
Brenowitz, E.A. Altered perception of species-specific song by female birds after lesions of a forebrain nucleus. Science 251, 303–305 (1991).
Yu, A.C. & Margoliash, D. Temporal hierarchical control of singing in birds. Science 273, 1871–1875 (1996).
Bottjer, S.W., Miesner, E.A. & Arnold, A.P. Forebrain lesions disrupt development, but not maintenance of song in passerine birds. Science 224, 901–903 (1984).
Hahnloser, R.H., Kozhevnikov, A.A. & Fee, M.S. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419, 65–70 (2002).
Prather, J.F., Peters, S., Nowicki, S. & Mooney, R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature 451, 305–310 (2008).
Mooney, R., Hoese, W. & Nowicki, S. Auditory representation of the vocal repertoire in a songbird with multiple song types. Proc. Natl. Acad. Sci. USA 98, 12778–12783 (2001).
Scharff, C., Nottebohm, F. & Cynx, J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J. Neurobiol. 36, 81–90 (1998).
Rizzolatti, G. & Craighero, L. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192 (2004).
Fee, M.S. & Leonardo, A. Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J. Neurosci. Methods 112, 83–94 (2001).
Studdert-Kennedy, M., Liberman, A.M., Harris, K.S. & Cooper, F.S. Theoretical notes. Motor theory of speech perception: a reply to Lane's critical review. Psychol. Rev. 77, 234–249 (1970).
Wyttenbach, R.A., May, M.L. & Hoy, R.R. Categorical perception of sound frequency by crickets. Science 273, 1542–1544 (1996).
Freedman, D.J., Riesenhuber, M., Poggio, T. & Miller, E.K. Categorical representation of visual stimuli in the primate prefrontal cortex. Science 291, 312–316 (2001).
Diehl, R.L., Lotto, A.J. & Holt, L.L. Speech perception. Annu. Rev. Psychol. 55, 149–179 (2004).
Balaban, E. Cultural and genetic variation in swamp sparrows (Melospiza georgiana). Behaviour 105, 250–290 (1988).
Eimas, P.D., Siqueland, E.R., Jusczyk, P. & Vigorito, J. Speech perception in infants. Science 171, 303–306 (1971).
Ballentine, B., Searcy, W.A. & Nowicki, S. Reliable aggressive signaling in swamp sparrows. Anim. Behav. 75, 693–703 (2008).
Eifuku, S., De Souza, W.C., Tamura, R., Nishijo, H. & Ono, T. Neuronal correlates of face identification in the monkey anterior temporal cortical areas. J. Neurophysiol. 91, 358–371 (2004).
Etcoff, N.L. & Magee, J.J. Categorical perception of facial expressions. Cognition 44, 227–240 (1992).
Marler, P. & Tamura, M. Culturally transmitted patterns of vocal behavior in sparrows. Science 146, 1483–1486 (1964).
Kuhl, P.K., Tsao, F.M. & Liu, H.M. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proc. Natl. Acad. Sci. USA 100, 9096–9101 (2003).
Freedman, D.J. & Assad, J.A. Experience-dependent representation of visual categories in parietal cortex. Nature 443, 85–88 (2006).
Coleman, M.J. & Mooney, R. Synaptic transformations underlying highly selective auditory representations of learned birdsong. J. Neurosci. 24, 7251–7265 (2004).
Rosen, M.J. & Mooney, R. Synaptic interactions underlying song-selectivity in the avian nucleus HVC revealed by dual intracellular recordings. J. Neurophysiol. 95, 1158–1175 (2006).
Rosen, M.J. & Mooney, R. Inhibitory and excitatory mechanisms underlying auditory responses to learned vocalizations in the songbird nucleus HVC. Neuron 39, 177–194 (2003).
Mooney, R. & Prather, J.F. The HVC microcircuit: the synaptic basis for interactions between song motor and vocal plasticity pathways. J. Neurosci. 25, 1952–1964 (2005).
Theunissen, F.E. & Doupe, A.J. Temporal and spectral sensitivity of complex auditory neurons in the nucleus HVc of male zebra finches. J. Neurosci. 18, 3786–3802 (1998).
Margoliash, D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J. Neurosci. 3, 1039–1057 (1983).
Margoliash, D. & Fortune, E.S. Temporal and harmonic combination-sensitive neurons in the zebra finch's HVc. J. Neurosci. 12, 4309–4326 (1992).
Lewicki, M.S. & Konishi, M. Mechanisms underlying the sensitivity of songbird forebrain neurons to temporal order. Proc. Natl. Acad. Sci. USA 92, 5582–5586 (1995).
Mooney, R. Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. J. Neurosci. 20, 5420–5436 (2000).
Author information
Authors and Affiliations
Contributions
J.F.P. collected and analyzed neural and behavioral data and wrote the manuscript. S.N. supervised the project, collected behavioral data and edited the manuscript. R.C.A. collected and analyzed behavioral data. S.P. collected and analyzed songs and created acoustic stimuli. R.M. supervised the project and wrote and edited the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Methods (PDF 1026 kb)
Rights and permissions
About this article
Cite this article
Prather, J., Nowicki, S., Anderson, R. et al. Neural correlates of categorical perception in learned vocal communication. Nat Neurosci 12, 221–228 (2009). https://doi.org/10.1038/nn.2246
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2246
This article is cited by
-
Categorical processing of fast temporal sequences in the guinea pig auditory brainstem
Communications Biology (2019)
-
Female cognitive performance and mass are correlated with different aspects of mate choice in the zebra finch (Taeniopygia guttata)
Animal Cognition (2019)
-
Cultural conformity generates extremely stable traditions in bird song
Nature Communications (2018)
-
A neuronal signature of accurate imitative learning in wild-caught songbirds (swamp sparrows, Melospiza georgiana)
Scientific Reports (2017)