Abstract
We developed a high-throughput mass spectrometry method, pLink-SS (http://pfind.ict.ac.cn/software/pLink/2014/pLink-SS.html), for precise identification of disulfide-linked peptides. Using pLink-SS, we mapped all native disulfide bonds of a monoclonal antibody and ten standard proteins. We performed disulfide proteome analyses and identified 199 disulfide bonds in Escherichia coli and 568 in proteins secreted by human endothelial cells. We discovered many regulatory disulfide bonds involving catalytic or metal-binding cysteine residues.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Alberts, B. et al. Molecular Biology of the Cell 3rd edn. 1, 978–993 (Garland Science, 1994).
Hogg, P.J. Trends Biochem. Sci. 28, 210–214 (2003).
van der Veen, A.G. & Ploegh, H.L. Annu. Rev. Biochem. 81, 323–357 (2012).
Monnier, V.M. et al. Ann. NY Acad. Sci. 1043, 533–544 (2005).
Tsai, P.L., Chen, S.-F. & Huang, S.Y. Rev. Anal. Chem. 32, 257–268 (2013).
Yang, B. et al. Nat. Methods 9, 904–906 (2012).
Zhang, W., Marzilli, L.A., Rouse, J.C. & Czupryn, M.J. Anal. Biochem. 311, 1–9 (2002).
Xu, H., Zhang, L. & Freitas, M.A. J. Proteome Res. 7, 138–144 (2008).
Depuydt, M., Messens, J. & Collet, J.F. Antioxid. Redox Signal. 15, 49–66 (2011).
Kadokura, H., Tian, H., Zander, T., Bardwell, J.C. & Beckwith, J. Science 303, 534–537 (2004).
Leichert, L.I. & Jakob, U. PLoS Biol. 2, e333 (2004).
Hiniker, A. & Bardwell, J.C. J. Biol. Chem. 279, 12967–12973 (2004).
Agudo, D., Mendoza, M.T., Castanares, C., Nombela, C. & Rotger, R. Proteomics 4, 355–363 (2004).
Vertommen, D. et al. Mol. Microbiol. 67, 336–349 (2008).
Zhu, J., Knottenbelt, S., Kirk, M.L. & Pei, D. Biochemistry 45, 12195–12203 (2006).
Hilgers, M.T. & Ludwig, M.L. Proc. Natl. Acad. Sci. USA 98, 11169–11174 (2001).
Fan, S.W. et al. Protein Sci. 18, 1745–1765 (2009).
Cook, K.M. & Hogg, P.J. Antioxid. Redox Signal. 18, 1987–2015 (2013).
Mishra, S. & Imlay, J. Arch. Biochem. Biophys. 525, 145–160 (2012).
Tam, J.P., Wu, C.R., Liu, W. & Zhang, J.W. J. Am. Chem. Soc. 113, 6657–6662 (1991).
Nossal, N.G. & Heppel, L.A. J. Biol. Chem. 241, 3055–3062 (1966).
Kosower, N.S. & Kosower, E.M. Methods Enzymol. 251, 123–133 (1995).
Wu, S.L. et al. Anal. Chem. 81, 112–122 (2009).
Xu, T. et al. Mol. Cell. Proteomics 5, S174 (2006).
Tabb, D.L., McDonald, W.H. & Yates, J.R. J. Proteome Res. 1, 21–26 (2002).
Yuan, Z.F. et al. Proteomics 12, 226–235 (2012).
Götze, M. et al. J. Am. Soc. Mass Spectrom. 23, 76–87 (2012).
Pandey, A., Andersen, J.S. & Mann, M. Sci. Signal. 2000, pl1 (2000).
Bassler, B.L., Wright, M. & Silverman, M.R. Mol. Microbiol. 13, 273–286 (1994).
Turovskiy, Y. & Chikindas, M.L. J. Microbiol. Methods 66, 497–503 (2006).
Surette, M.G. & Bassler, B.L. Proc. Natl. Acad. Sci. USA 95, 7046–7050 (1998).
Bindea, G. et al. Bioinformatics 25, 1091–1093 (2009).
Smoot, M.E., Ono, K., Ruscheinski, J., Wang, P.-L. & Ideker, T. Bioinformatics 27, 431–432 (2011).
Franceschini, A. et al. Nucleic Acids Res. 41, D808–D815 (2013).
Bader, G.D. & Hogue, C.W. BMC Bioinformatics 4, 2 (2003).
O'Shea, J.P. et al. Nat. Methods 10, 1211–1212 (2013).
Acknowledgements
We thank the National BioResource Project of Japan for providing bacterial strains; the antibody center of National Institute of Biological Sciences, Beijing, for the purified anti–DAF-16 IgG2 antibody; and X.-Z. Dong, A. Hühmer, D. Horn and Z. Hao for bringing the disulfide-bond problem to our attention and for encouragement. We also thank all members of the pFind group, X. Xiong, Y. Xia, S. Chen, K. Ye, Y. Zhou and members of the Dong lab for discussion and experimental support. This work was funded by the National Scientific Instrumentation Grant Program (2011YQ09000506 to M.-Q.D.), National Natural Science Foundation of China (grant no. 21475141 to S.-M.H.), Ministry of Science and Technology of China (973 grants 2013CB911203, 2012CB910602 to R.-X.S. and 2010CB912701 to S.-M.H.), CAS Knowledge Innovation Program (grant #KGCX1-YW-13 and ICT-20126033 to S.-M.H.), Strategic Priority Research Program of CAS (XDB13040600 to Y.F.), NCMIS CAS and municipal government of Beijing.
Author information
Authors and Affiliations
Contributions
S.L. and B.Y. performed experiments, analyzed data and prepared the manuscript; S.-B.F. analyzed data and developed pLink-SS; J.-M.M., L.W., K.Z., Y.F. and R.-X.S. helped software development and data analysis; Y.-X.L. performed statistical analysis, pathway analysis and protein network analysis; M.-J.Z. helped with informatics analysis; P.L. and J.L. prepared the HUVEC samples; S.-M.H. directed software development and edited the manuscript; M.-Q.D. directed wet-lab experiments and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 HCD outperformed electron-transfer dissociation (ETD) in identification of disulfide-linked synthetic peptides.
(a) Summary of identification results from paired HCD and ETD MS2 spectra of disulfide-linked synthetic peptides of known sequences. A total of 1398 non-redundant disulfide-bonded peptide pairs were identified, of which 986 were identified only by HCD and 27 only by ETD. The data were searched using pLink by considering a1+, a2+, b1+, b2+, y1+ and y2+ ions for HCD spectra, c1+, c2+, [c-H]1+, [c-H]2+, z1+, z2+, [z+H]1+, and [z+H]2+ ions for ETD spectra, and filtered by requiring E-value < 0.01 for HCD matches and E-value < 1 for ETD results, respectively. The E-value is calculated by multiplying the p-value of a match, which estimates how likely a candidate cross-link matches to a spectrum by random chance, by the number of candidate cross-links falling within the mass tolerance window in the searched database. (b-d) For +3 and +4 precursors, the fragmentation efficiency is low for ETD compared with HCD. In this collection, there are 1162, 754, 151 and 54 ETD/HCD spectral pairs for +3, +4, +5 and +6 precursor ions, respectively. (b) In ETD spectra, the relative intensity of remaining precursor ions is high, especially for +3 and +4 precursors. (c) For many precursors, the intensity of peptide ions (p ions, due to disulfide bond cleavage) is too low (near or at zero percent) for peptide sequencing by MS3. (d) The relative intensity of all the ions found in an ETD or HCD spectrum that are neither precursor ions nor p ions.
Supplementary Figure 2 Fragment-ion types that are specific for disulfide-linked peptides and their contribution to disulfide bond identification.
(a-b) Illustration of the disulfide bond-specific fragment ions found in HCD spectra, including p ions (αp, bp, αp(-2), bp(-2), αp(+32), bp(+32), αp(-34) and βp(-34)), pb and py ions (e.g. αp(-2)b4 and αp(-2)y4). (c) An HCD spectrum in which disulfide bond-specific fragment ions are found (inset). (d) E-value separation of correct and random matches of disulfide-bonded peptides when different ion types are considered in the pLink search. The test data consisted of 1141 HCD spectra of disulfide-linked peptide pairs whose identities were known and 46526 HCD spectra of reduced and alkylated linear peptides from a trypsin-digested E. coli lysate. Solid and dashed lines denote forward (pos) and reverse (neg) database hits, respectively.
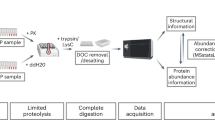
Supplementary Figure 3 Nonspecific protease digestion allowed the identification of all native disulfide bonds in RNase A.
Predicted digestion products are shown above the experimental results. Cross mark indicates that those complex forms cannot be identified.
Supplementary Figure 5 Representative HCD spectra showing the identification of two disulfide bonds in the variable regions of a mouse monoclonal antibody (anti–DAF-16, IgG2).
LC, light chain; HC, heavy chain.
Supplementary Figure 7 Mapping of disulfide bonds in a ten-protein mixture.
(a) Properties of ten standard proteins. (b) Verification of the sample digestion condition optimized on RNase A. Samples were digested with Lys-C/Trypsin/Glu-C in this set of experiments. (c) All 74 disulfide bonds were identified using different proteases. (d) There are unique identifications in each digestion. (e) Some of the disulfide bonds were identified in different forms.
Supplementary Figure 9 Specific proteases outperformed nonspecific proteases in disulfide-bond analysis of complex protein samples.
(a) Number of disulfide bonds identified from an E. coli periplasmic fraction using different proteases. Number of unique identifications is shown in parentheses. (b) The search space and time cost for each protease digestion tested. Similar amounts of HCD spectra were collected from different protease digestions, and they were all searched against the same E. coli database using the same hardware setup (computer cluster, 6 units of Dual Xeon 5405, 2.0 GHz, 4G RAM, 8 core/unit) and the same pLink-SS parameters except for the protease specificity setting.
Supplementary Figure 10 Interprotein and intraprotein disulfide bonds identified from complex samples are filtered separately.
(a) More than 95% of the disulfide bonds recorded in E. coli, C. elegans and human protein databases are intra-protein disulfide bonds. (b) Violin plots showing the number and E-value distributions of forward-forward, forward-reverse/reverse-forward, and reverse-reverse matches in the unfiltered pLink-SS search result of a Lys-C/Trypsin/Glu-C digested E. coli periplasmic fraction. Top panel: inter-protein matches; bottom panel: intra-protein matches. (c) The formula and the basis of FDR calculation for intra-protein disulfide-bond identifications. The HCD spectra of the ten standard proteins (Supplementary Fig. 5), of which none is an E. coli or Crenarchaeota protein, were searched against an E. coli or Crenarchaeota protein database. Thus, all matches are random. The match results were not filtered in any way. It shows that for intra-protein results, random spectral matches to forward-forward (F-F) and reverse-reverse (R-R) sequence pairs are essentially the same in number, thus the number of R-R matches can be used to estimate the FDR. (d) The E-value distributions of the 57 F-F and 57 R-R random matches with an E-value < 1 (a built-in threshold for pLink-SS to output a match) have no significant difference (p = 0.91, two-sample Kolmogorov-Smirnov test, same below). These were filtered from the random matches obtained by searching the 176,500 spectra in (c) against the same E. coli database digested in silico with trypsin. The same conclusion was reached using Lys-C/Trypsin/Glu-C for in silico digestion (41 F-F vs. 50 R-R random hits with E-value < 1, p = 0.11). (e) The E-value distributions of the 203 F-F and 172 R-R random matches obtained by searching the 176,500 spectra against an artificial protein sequence database. The requirement of E-value < 1 was lifted. There is no statistical difference between the two empirical cumulative distribution curves (p = 0.29, two-sample Kolmogorov-Smirnov test). This again validates the FDR calculation formula for intra-protein disulfide-bonds in (c).
Supplementary Figure 11 Known and novel protein disulfide bonds identified in the E. coli periplasmic fraction.
(a) Known (black) and newly identified (red) disulfide bond-containing proteins are grouped according to their annotated function and subcellular localization. (b) Classification of the identified E. coli disulfide bonds into consecutive, non-consecutive, and inter-protein disulfide bonds. Two disulfide bonds identified in a complex form cannot be determined as consecutive or non-consecutive.
Supplementary Figure 12 Eight purified E. coli proteins and the verification results of their disulfide bonds using undigested proteins.
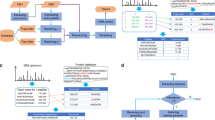
(a) SDS-PAGE of purified His6-tagged proteins. (b) MS1 spectra showing a mass difference of 252.5 Da between the reduced and unreduced E. coli protein EfeO. (c) Summary of the disulfide bond verification results from protein analysis as outlined in Figure 2a.
Supplementary Figure 13 The formation of the intersubunit disulfide bonds of LuxS is redox sensitive and may affect AI-2 production.
(a) Crystal structure of a LuxS homo-dimer (subunits depicted as blue and green ribbons) of Bacillus subtilis showing that the intra-subunit distance between C84 and C126 (corresponding to C83 and C128 of E. coli LuxS) is far but the inter-subunit distance is short. (b) Reductant-sensitive WT E. coli LuxS dimers. MS analysis identified C83-C128 in the upper and C41-C128 in the lower band. Mutating C83 or C128 into serine abolished one or both bands. (c) The amount of disulfide-linked LuxS dimers increased following a 15-min H2O2 treatment and decreased after H2O2 was removed. (d) The increase in LuxS dimer formation was accompanied by a decrease in AI-2 production.
Supplementary Figure 14 Changes in protein disulfide bonds and glutathionylation in human A549 cells upon induction of oxidative stress.
(a) More protein cysteine residues were found in the oxidized forms upon 1 mM diamide treatment for 10 min, including protein disulfide bonds and glutathionylated forms. (b) Of the known disulfide bond-containing proteins, most of disulfide bonds identified in A549 cells agree with the database annotations. (c) A Cytoscape view of the densely connected subnetworks of the proteins that had increased levels of disulfide bonds or glutathionylation after the diamide treatment. The subnetwork with the highest MCODE complex score, defined as the product of the density and the number of nodes in the detected complex, is highlighted and shown on the right. Proteins functioning in mRNA processing are colored red. (d) The three most significantly enriched pathways among the proteins that had increased levels of disulfide bonds or glutathionylation after 1 mM diamide treatment. The p values were calculated using a right-sided hypergeometrical test and corrected for multiple testing using the Benjamini-Hochberg method. (e) Of the proteins that appear to be readily glutathionylated are seven oxidoreductases (red) and two proteins involved in F-actin formation or depolymerization (orange). MGST1: Microsomal glutathione S-transferase 1, CPLX2: Complexin-2, PBK: Lymphokine-activated killer T-cell-originated protein kinase, TXN: Thioredoxin, CPSF6: Cleavage and polyadenylation specificity factor subunit 6, PRDX5: Mitochondrial peroxiredoxin-5, RPLP1: 60S acidic ribosomal protein P1, PRDX3: Mitochondrial thioredoxin-dependent peroxide reductase, DSTN: Actin-depolymerizing factor, PRDX1: Peroxiredoxin-1, CFL1: Cofilin-1, PCBP1: Poly(rC)-binding protein 1, EIF5A: Eukaryotic translation initiation factor 5A-1, AKR1C1: Aldo-keto reductase family 1 member C1, AKR1C3: Aldo-keto reductase family 1 member C3. (f) Motif analysis of glutathionylation sites using pLogo (http://plogo.uconn.edu). The number of aligned foreground and background sequences are 1697 and 12822, respectively. The red horizontal bars correspond to p = 0.05.
Supplementary Figure 15 Enrichment of the CXXC-type disulfide bonds in E. coli.
Disulfide bonds identified from E. coli (a) or human cells (b) are binned based on the number of animo acids separating the two disulfide-forming cysteine residues. For all the proteins in the E. coli or human protein database, any two adjacent cysteine residues are also binned according to the number of animo acids between them. In E. coli, the percentage of CXXC among the identified disulfide bonds is significantly higher than what might be expected from the whole proteome (p < 0.001, Pearson’s Chi-squared test). This was not seen in A549 or HUVEC cells under normal conditions. Only in diamide-treated A549 cells was there an enrichment of CXXC-type disulfide bonds (p < 0.001, Pearson’s Chi-squared test).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and Supplementary Tables 1, 2 and 10 (PDF 7154 kb)
Supplementary Table 3
Disulfide bonds identified from the E. coli periplasmic fraction (XLS 104 kb)
Supplementary Table 4
Potential substrates of DsbA (XLS 51 kb)
Supplementary Table 5
Potential substrates of DsbC (XLS 31 kb)
Supplementary Table 6
Disulfide bonds identified in human A549 cells (XLS 199 kb)
Supplementary Table 7
Glutathionylation sites identified in human A549 cells (XLS 493 kb)
Supplementary Table 8
Disulfide bonds identified in secreted HUVEC proteins (XLS 258 kb)
Supplementary Table 9
Candidate allosteric disulfide bonds in HUVEC proteins (XLS 31 kb)
Rights and permissions
About this article
Cite this article
Lu, S., Fan, SB., Yang, B. et al. Mapping native disulfide bonds at a proteome scale. Nat Methods 12, 329–331 (2015). https://doi.org/10.1038/nmeth.3283
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.3283
This article is cited by
-
Pathway and mechanism of tubulin folding mediated by TRiC/CCT along its ATPase cycle revealed using cryo-EM
Communications Biology (2023)
-
A quantitative interpretation of oxidative protein folding activity in Escherichia coli
Microbial Cell Factories (2022)
-
Structural insights into dsRNA processing by Drosophila Dicer-2–Loqs-PD
Nature (2022)
-
Efficient preparation of unsymmetrical disulfides by nickel-catalyzed reductive coupling strategy
Nature Communications (2022)
-
Unsymmetrical polysulfidation via designed bilateral disulfurating reagents
Nature Communications (2020)