Abstract
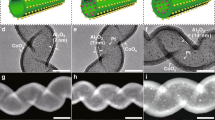
Tuning the electronic structure of heterogeneous metal catalysts has emerged as an effective strategy to optimize their catalytic activities. By preparing ethylenediamine-coated ultrathin platinum nanowires as a model catalyst, here we demonstrate an interfacial electronic effect induced by simple organic modifications to control the selectivity of metal nanocatalysts during catalytic hydrogenation. This we apply to produce thermodynamically unfavourable but industrially important compounds, with ultrathin platinum nanowires exhibiting an unexpectedly high selectivity for the production of N-hydroxylanilines, through the partial hydrogenation of nitroaromatics. Mechanistic studies reveal that the electron donation from ethylenediamine makes the surface of platinum nanowires highly electron rich. During catalysis, such an interfacial electronic effect makes the catalytic surface favour the adsorption of electron-deficient reactants over electron-rich substrates (that is, N-hydroxylanilines), thus preventing full hydrogenation. More importantly, this interfacial electronic effect, achieved through simple organic modifications, may now be used for the optimization of commercial platinum catalysts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sheldon, R. A. & Van Bekkum, H. Fine Chemicals Through Heterogeneous Catalysis (John Wiley, 2008).
Noyori, R. Synthesizing our future. Nature Chem. 1, 5–6 (2009).
Somorjai, G. A. & Rioux, R. M. High technology catalysts towards 100% selectivity fabrication, characterization and reaction studies. Catal. Today 100, 201–215 (2005).
Van Leeuwen, P. W. Homogeneous Catalysis: Understanding the Art (Springer Science Business Media, 2006).
Schmid, A. et al. Industrial biocatalysis today and tomorrow. Nature 409, 258–268 (2001).
Rod, T. H. & Norskov, J. K. The surface science of enzymes. Surf. Sci. 500, 678–698 (2002).
Wang, D.-H., Engle, K. M., Shi, B.-F. & Yu, J.-Q. Ligand-enabled reactivity and selectivity in a synthetically versatile aryl C–H olefination. Science 327, 315–319 (2010).
Swiegers, G. F. Mechanical Catalysis: Methods of Enzymatic, Homogeneous, and Heterogeneous Catalysis (John Wiley, 2008).
Bhaduri, S. & Mukesh, D. Homogeneous Catalysis: Mechanisms and Industrial Applications (Wiley, 2000).
Marshall, S. T. et al. Controlled selectivity for palladium catalysts using self-assembled monolayers. Nature Mater. 9, 853–858 (2010).
Astruc, D., Lu, F. & Aranzaes, J. R. Nanoparticles as recyclable catalysts. The frontier between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 44, 7852–7872 (2005).
Wu, B. H. & Zheng, N. F. Surface and interface control of noble metal nanocrystals for catalytic and electrocatalytic applications. Nano Today 8, 168–197 (2013).
Medlin, J. W. Controlling selectivity in heterogeneous catalysis with organic modifiers. Acc. Chem. Res. 47, 1438–1445 (2013).
Jones, S., Qu, J., Tedsree, K., Gong, X.-Q. & Tsang, S. C. E. Prominent electronic and geometric modifications of palladium nanoparticles by polymer stabilizers for hydrogen production under ambient conditions. Angew. Chem. Int. Ed. 51, 11275–11278 (2012).
Luksirikul, P., Tedsree, K., Moloney, M. G., Green, M. L. H. & Tsang, S. C. E. Electron promotion by surface functional groups of single wall carbon nanotubes to overlying metal particles in a fuel-cell catalyst. Angew. Chem. Int. Ed. 51, 6998–7001 (2012).
Stamenkovic, V. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 45, 2897–2901 (2006).
Stamenkovic, V. R. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Zhang, J., Sasaki, K., Sutter, E. & Adzic, R. R. Stabilization of platinum oxygen-reduction electrocatalysts using gold clusters. Science 315, 220–222 (2007).
Chen, C. et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 343, 1339–1343 (2014).
Cui, C. et al. Octahedral PtNi nanoparticle catalysts: exceptional oxygen reduction activity by tuning the alloy particle surface composition. Nano Lett. 12, 5885–5889 (2012).
Strasser, P. et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nature Chem. 2, 454–460 (2010).
Kim, D., Resasco, J., Yu, Y., Asiri, A. M. & Yang, P. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold–copper bimetallic nanoparticles. Nature Commun. 5, 4948 (2014).
Tedsree, K. et al. Hydrogen production from formic acid decomposition at room temperature using a Ag–Pd core-shell nanocatalyst. Nature Nanotech. 6, 302–307 (2011).
Enache, D. I. et al. Solvent-free oxidation of primary alcohols to aldehydes using Au–Pd/TiO2 catalysts. Science 311, 362–365 (2006).
Zhang, H., Watanabe, T., Okumura, M., Haruta, M. & Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nature Mater. 11, 49–52 (2012).
Chan, C. W. A. et al. Interstitial modification of palladium nanoparticles with boron atoms as a green catalyst for selective hydrogenation. Nature Commun. 5, 5787 (2014).
Vayssilov, G. N. et al. Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nature Mater. 10, 310–315 (2011).
Campbell, C. T. Catalyst-support interactions: electronic perturbations. Nature Chem. 4, 597–598 (2012).
Bell, A. T. The impact of nanoscience on heterogeneous catalysis. Science 299, 1688–1691 (2003).
Ross, J. R. Heterogeneous Catalysis: Fundamentals and Applications (Elsevier, 2012).
Blaser, H.-U. A golden boost to an old reaction. Science 313, 312–313 (2006).
Corma, A. & Serna, P. Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313, 332–334 (2006).
Solomina, T. A. et al. Prospects of catalytic reduction of aromatic nitro compounds by hydrogen. Int. J. Hydrog. Energy 20, 159–161 (1995).
Bordwell, F. G. & Liu, W.-Z. Equilibrium acidities and homolytic bond dissociation energies of N–H and/or O–H bonds in N-phenylhydroxylamine and its derivatives. J. Am. Chem. Soc. 118, 8777–8781 (1996).
Evans, D. A., Song, H.-J. & Fandrick, K. R. Enantioselective nitrone cycloadditions of alpha, beta-unsaturated 2-acyl imidazoles catalyzed by bis(oxazolinyl)pyridine-cerium(IV) triflate complexes. Org. Lett. 8, 3351–3354 (2006).
Sun, Z. Y. et al. The solvent-free selective hydrogenation of nitrobenzene to aniline: an unexpected catalytic activity of ultrafine Pt nanoparticles deposited on carbon nanotubes. Green Chem. 12, 1007–1011 (2010).
Corma, A., Serna, P., Concepcion, P. & Calvino, J. J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 130, 8748–8753 (2008).
Möbus, K. et al. Hydrogenation of aromatic nitrogroups with precious metal powder catalysts: influence of modifier on selectivity and activity. Top. Catal. 53, 1126–1131 (2010).
Takasaki, M. et al. Chemoselective hydrogenation of nitroarenes with carbon nanofiber-supported platinum and palladium nanoparticles. Org. Lett. 10, 1601–1604 (2008).
Linstrom, P. J. & Mallard, W. G. (eds) NIST Chemistry WebBook, NIST Standard Reference Database Number 69 (National Institute of Standards and Technology, 19 December 2015); http://webbook.nist.gov
Oxley, P. W., Adger, B. M., Sasse, M. J. & Forth, M. A. N-acetyl-N-phenylhydroxylamine via catalytic transfer hydrogenation of nitrobenzene using hydrazine and rhodium on carbon. Org. Synth. http://doi.org/bmp73h (2003).
Takenaka, Y., Kiyosu, T., Choi, J.-C., Sakakura, T. & Yasuda, H. Selective synthesis of N-aryl hydroxylamines by the hydrogenation of nitroaromatics using supported platinum catalysts. Green Chem. 11, 1385–1390 (2009).
Kinomoto, Y., Watanabe, S., Takahashi, M. & Ito, M. Infrared spectra of CO adsorbed on Pt(100), Pt(111), and Pt(110) electrode surfaces. Surf. Sci. 242, 538–543 (1991).
Owen, J. The coordination chemistry of nanocrystal surfaces. Science 347, 615–616 (2015).
CrysAlis Pro Version 1.171.35.19 (Agilent Technologies, 2011).
Sheldrick, G. M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. C 71, 3–8 (2015).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 48, 13115–13118 (1993).
Frisch, M. J. et al. Gaussian 09, Revision D.01 (Gaussian, 2009).
Galano, A. & Cruz-Torresb, A. OH radical reactions with phenylalanine in free and peptide forms. Org. Biomol. Chem. 6, 732–738 (2008).
Acknowledgements
We thank the MOST of China (2015CB932303), the NSFC (21420102001, 21131005, 21390390, 21333008, 21373167, 21133004), IRT_14R31, and the NFFTBS (J1210014) for the financial support. We thank Y. P. Zheng for help with XPS measurements, S. Q. Wei for preliminary XAS tests, Y. Ding and Z. L. Wang for preliminary HRTEM measurements. We also thank L. S. Zheng, Z. Q. Tian, L. W. Ye., M. S. Chen, B. Ren, J. F. Li, P. Zhang, Y. Zhao, J. Yang and P. N. Duchesne for helpful discussions.
Author information
Authors and Affiliations
Contributions
N.Z. conceived the research project. G.C., C.X., X.H., Z.Z. and B.W. designed and synthesized the nanomaterials and carried out the catalysis experiments. G.F. carried out the model construction and DFT calculations. G.L. and Z.T. performed the TPD-MS experiments. J.Y. and Z.Z. carried out the in situ FTIR spectroscopy experiments. L.G. performed the HRTEM measurements. H.Y. performed the single-crystal analysis. All the authors contributed to the data analysis and drafted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4037 kb)
Rights and permissions
About this article
Cite this article
Chen, G., Xu, C., Huang, X. et al. Interfacial electronic effects control the reaction selectivity of platinum catalysts. Nature Mater 15, 564–569 (2016). https://doi.org/10.1038/nmat4555
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat4555
This article is cited by
-
Carbon–carbon bond cleavage for a lignin refinery
Nature Chemical Engineering (2024)
-
Doping engineering of iron oxide nanoparticles towards high performance and biocompatible T1-weighted MRI contrast agents
Rare Metals (2024)
-
UIO-66-NH2-Derived Porous Carbon Doping with Nitrogen-Supported Pt as an Electrocatalyst for Hydrogen Evolution Reaction
Catalysis Letters (2024)
-
Ultrathin covalent organic overlayers on metal nanocrystals for highly selective plasmonic photocatalysis
Nature Communications (2023)
-
Solvent-free synthesis of Co@NC catalyst with Co—N species as active sites for chemoselective hydrogenation of nitro compounds
Science China Materials (2023)