Abstract
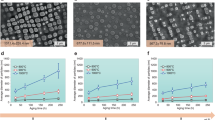
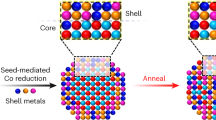
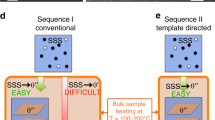
The size distribution of particles, which is essential for many properties of nanomaterials, is equally important for the mechanical behaviour of the class of alloys whose strength derives from a dispersion of nanoscale precipitates. However, particle size distributions formed by solid-state precipitation are generally not well controlled. Here we demonstrate, through the example of core–shell precipitates in Al–Sc–Li alloys, an approach to forming highly monodisperse particle size distributions by simple solid-state reactions. The approach involves the use of a two-step heat treatment, whereby the core formed at high temperature provides a template for growth of the shell at lower temperature. If the core is allowed to grow to a sufficient size, the shell develops in a ‘size focusing’ regime, where smaller particles grow faster than larger ones. These results suggest strategies for manipulating precipitate size distributions in similar systems through simple variations in thermal treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gayle, F. & Goodway, M. Precipitation hardening in the first aerospace aluminum-alloy—the Wright Flyer crankcase. Science 266, 1015–1017 (1994).
Lu, K. The future of metals. Science 328, 319–320 (2010).
Mao, Z., Sudbrack, C. K., Yoon, K. E., Martin, G. & Seidman, D. N. The mechanism of morphogenesis in a phase-separating concentrated multicomponent alloy. Nature Mater. 6, 210–216 (2007).
Clouet, E. et al. Complex precipitation pathways in multicomponent alloys. Nature Mater. 5, 482–488 (2006).
Sigli, C. Aluminium Alloys: Their Physical and Mechanical Properties 50–59 (Wiley–VCH, 2008).
Ardell, A. & Ozolins, V. Trans-interface diffusion-controlled coarsening. Nature Mater. 4, 309–316 (2005).
Srinivasan, R. et al. Atomic scale structure and chemical composition across order–disorder interfaces. Phys. Rev. Lett. 102, 86101 (2009).
Marquis, E. A., Seidman, D. N., Asta, M., Woodward, C. & Ozolins, V. Mg segregation at Al/Al3Sc heterophase interfaces on an atomic scale: Experiments and computations. Phys. Rev. Lett. 91, 036101 (2003).
Miura, Y., Horikawa, K., Yamada, K. & Nakayama, M. in Aluminum Alloys: Their Physical and Mechanical Properties, Papers Presented at the International Conference, 4th, Atlanta, Sept. 11–16, 1994 (1994), Vol. 2 (eds Sanders, T. H. & Starke, E. A.) 161–168 (Georgia Institute of Technology, 1994).
Berezina, A. L., Kolobnev, N. I., Chuistov, K. V., Kotko, A. B. & Molebny, O. A. Coherent composite phases formation in aged Al–Li base alloys. Mater. Sci. Forum 396–402, 977–982 (2002).
Krug, M. E., Dunand, D. C. & Seidman, D. N. Composition profiles within Al3Li and Al3Sc/Al3Li nanoscale precipitates in aluminum. Appl. Phys. Lett. 92, 124107 (2008).
Krug, M. E., Dunand, D. C. & Seidman, D. N. Effects of Li additions on precipitation-strengthened Al–Sc and Al–Sc–Yb alloys. Acta Mater. 59, 1700–1715 (2011).
Radmilovic, V., Tolley, A., Marquis, E. A., Rossell, M. D., Lee, Z. & Dahmen, U. Monodisperse Al3(LiScZr) core/shell precipitates in Al alloys. Scr. Mater. 58, 529–532 (2008).
Hyland, R. W. Homogeneous nucleation kinetics of Al3Sc in a dilute Al–Sc alloy. Metall. Mater. Trans. A 23, 1947–1955 (1992).
Radmilovic, V., Fox, A. G. & Thomas, G. Spinodal decomposition of Al-rich Al–Li alloys. Acta Metall. 37, 2385–2394 (1989).
Fox, A. G., Fuller, S. C., Radmilovic, V. & Whitman, C. A powder x-ray diffraction study of a solution treated and ice brine quenched Al–14.25 at.% Li alloy. J. Mater. Res. 6, 712–718 (1991).
Yin, Y. & Alivisatos, A. P. Colloidal nanocrystal synthesis and the organic–inorganic interface. Nature 437, 664–670 (2005).
Clouet, E., Barbu, A., Laé, L. & Martin, G. Precipitation kinetics of A13Zr and Al3Sc in aluminum alloys modeled with cluster dynamics. Acta Mater. 53, 2313–2325 (2005).
Robson, J. D. & Prangnell, P. B. Modelling Al3Zr dispersoid precipitation in multicomponent aluminium alloys. Mater. Sci. Eng. A 352, 240–250 (2003).
Tolley, A., Radmilovic, V. & Dahmen, U. Segregation in Al3(Sc,Zr) precipitates in Al–Sc–Zr alloys. Scr. Mater. 52, 621–625 (2005).
Aydinol, M. K. & Bor, A. S. Coarsening of δ′ (Al3 Li) and composite precipitates in an Al-2.5-% Li-0.15-% Zr alloy. J. Mater. Sci. 29, 15–25 (1994).
Kuehmann, C. J. & Voorhees, P. W. Ostwald ripening in ternary alloys. Metall. Mater. Trans. A 27, 937–943 (1996).
Moreau, C., Allouche, A. & Knystautas, E. J. Measurements of the diffusion rate of lithium in aluminum at low-temperature by elastic recoil detection analysis. J. Appl. Phys. 58, 4582–4586 (1985).
Fujikawa, S. I. Impurity diffusion of scandium in aluminium. Defect Diffusion Forum 143–147, 115–120 (1997).
Royset, J. & Ryum, N. Kinetics and mechanisms of precipitation in an Al-0.2 wt.% Sc alloy. Mater. Sci. Eng. A 396, 409–422 (2005).
Noble, B. & Bray, S. E. Use of the Gibbs–Thompson relation to obtain the interfacial energy of δ′ precipitates in Al–Li alloys. Mater. Sci. Eng. A 266, 80–85 (1999).
Murray, J. The Al–Sc system. J. Phase Equilib. 19, 380–384 (1998).
Hallstedt, B. & Kim, O. Thermodynamic assessment of the Al–Li system. Int. J. Mater. Res. 98, 961–969 (2007).
Mao, Z., Chen, W., Seidman, D. N. & Wolverton, C. First-principles study of the nucleation and stability of ordered precipitates in ternary Al–Sc–Li alloys. Acta Mater. 59, 3012–3023 (2011).
Voorhees, P. W. & Johnson, W. C. Development of spatial correlations during diffusional late-stage phase transformations in stressed solids. Phys. Rev. Lett. 61, 2225–2228 (1988).
LaMer, V. K. & Dinegar, R. H. Theory, production and mechanism of formation of monodispersed hydrosols. J. Am. Chem. Soc. 72, 4847–4854 (1950).
Monachon, C., Dunand, D. C. & Seidman, D. N. Atomic-scale characterization of aluminum-based multishell nanoparticles created by solid-state synthesis. Small 6, 1728–1731 (2010).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Hafner, J. Ab-initio molecular-dynamics for liquid-metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab-initio molecular-dynamics simulation of the liquid-metal amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169 (1996).
Van de Walle, A., Asta, M. & Ceder, G. M. The alloy theoretic automated toolkit: A user guide. Calphad 26, 539–553 (2002).
Acknowledgements
This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, Materials Science and Engineering Division of the US Department of Energy under Contracts # DE-AC02-05CH11231 (V.R., A.T., A.G., U.D.) and DE-FG02-06ER46282 (M.A.). Electron microscopy was performed at the National Center for Electron Microscopy, which is supported by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract No. DE-AC02-05CH11231. C.O. acknowledges funding from the National Sciences and Engineering Research Council of Canada. V.R. acknowledges support of Nanotechnology and Functional Materials Center, funded by the European FP7 project No. 245916, and support from the Ministry of Education and Science of the Republic of Serbia, under project No. 172054. M. Watanabe, R. Erni and Z. Lee are acknowledged for their assistance to M.D.R. in TEM/STEM/EELS data acquisition/reconstruction. We acknowledge Mr. J. Wu of LBNL, Materials Science Division, for alloy preparation.
Author information
Authors and Affiliations
Contributions
V.R. conceived and designed the experiments and wrote the first draft of the manuscript. U.D., M.A. and C.O. co-wrote the manuscript. C.O. and M.A. performed first-principles simulation and continuum modelling. M.D.R. and V.R. carried out high-resolution microscopy and exit wave reconstruction. E.A.M. performed 3D-APT experiments and analysis. A.T., M.D.R. and A.G. carried out sample preparation and basic TEM characterization. V.R., U.D., M.A. and C.O. analysed the experimental results. All authors contributed to discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 1284 kb)
Rights and permissions
About this article
Cite this article
Radmilovic, V., Ophus, C., Marquis, E. et al. Highly monodisperse core–shell particles created by solid-state reactions. Nature Mater 10, 710–715 (2011). https://doi.org/10.1038/nmat3077
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3077
This article is cited by
-
Probing the atomically diffuse interfaces in Pd@Pt core-shell nanoparticles in three dimensions
Nature Communications (2023)
-
On the microstructural characteristics and mechanical properties of Al−2Li−2Cu−0.5 Mg alloy: the role of Yb additions
Journal of Materials Science (2022)
-
Effect of Single-Pass Large-Strain Rolling on Microstructure and Mechanical Properties of Al-3Li-1Cu-0.2Er-0.1Zr Alloy
Journal of Materials Engineering and Performance (2022)
-
Microalloying Al alloys with Sc: a review
Rare Metals (2020)
-
Equilibrium Multi-precipitate Configurations
Metallurgical and Materials Transactions A (2020)