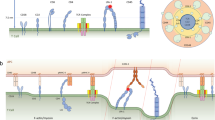
Abstract
During activation, T cells associate with antigen-presenting cells, a dynamic process that involves the formation of a broad area of intimate membrane contact known as the immunological synapse. The molecular intermediates that link initial antigen recognition to the cytoskeletal changes involved in this phenomenon have not yet been defined. Here we demonstrate that ezrin-radixin-moesin proteins are rapidly inactivated after antigen recognition through a Vav1-Rac1 pathway. The resulting disanchoring of the cortical actin cytoskeleton from the plasma membrane decreased cellular rigidity, leading to more efficient T cell–antigen-presenting cell conjugate formation. These findings identify an antigen-dependent molecular pathway that favors immunological synapse formation and the subsequent development of an effective immune response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
09 February 2004
appended aop PDF with erratum PDF (will be corrected for print issue), and placed footnote in SGML at all occurrences of Figure 2
References
Bromley, S.K. et al. The immunological synapse. Annu. Rev. Immunol. 19, 375–396 (2001).
Lanzavecchia, A. & Sallusto, F. Antigen decoding by T lymphocytes: from synapses to fate determination. Nat. Immunol. 2, 487–492 (2001).
Delon, J., Stoll, S. & Germain, R.N. Imaging of T-cell interactions with antigen presenting cells in culture and in intact lymphoid tissue. Immunol. Rev. 189, 51–63 (2002).
Huppa, J.B. & Davis, M.M. T-cell-antigen recognition and the immunological synapse. Nat. Rev. Immunol. 3, 973–983 (2003).
Trautmann, A. & Valitutti, S. The diversity of immunological synapses. Curr. Opin. Immunol. 15, 249–254 (2003).
Dustin, M.L., Bromley, S.K., Kan, Z., Peterson, D.A. & Unanue, E.R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA 94, 3909–3913 (1997).
Delon, J., Bercovici, N., Liblau, R. & Trautmann, A. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur. J. Immunol. 28, 716–729 (1998).
Stoll, S., Delon, J., Brotz, T.M. & Germain, R.N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Donnadieu, E., Bismuth, G. & Trautmann, A. Antigen recognition by helper T cells elicits a sequence of distinct changes of their shape and intracellular calcium. Curr. Biol. 4, 584–595 (1994).
Negulescu, P.A., Krasieva, T.B., Khan, A., Kerschbaum, H.H. & Cahalan, M.D. Polarity of T cell shape, motility, and sensitivity to antigen. Immunity 4, 421–430 (1996).
Wülfing, C., Sjaastad, M.D. & Davis, M.M. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA 95, 6302–6307 (1998).
Delon, J., Bercovici, N., Raposo, G., Liblau, R. & Trautmann, A. Antigen-dependent and -independent Ca2+ responses triggered in T cells by dendritic cells compared with B cells. J. Exp. Med. 188, 1473–1484 (1998).
Valitutti, S., Dessing, M., Aktories, K., Gallati, H. & Lanzavecchia, A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J. Exp. Med. 181, 577–584 (1995).
Dustin, M.L. & Cooper, J.A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1, 23–29 (2000).
Sancho, D. et al. Regulation of microtubule-organizing center orientation and actomyosin cytoskeleton rearrangement during immune interactions. Immunol. Rev. 189, 84–97 (2002).
Fuller, C.L., Braciale, V.L. & Samelson, L.E. All roads lead to actin: the intimate relationship between TCR signaling and the cytoskeleton. Immunol. Rev. 191, 220–236 (2003).
Allenspach, E.J. et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity 15, 739–750 (2001).
Delon, J., Kaibuchi, K. & Germain, R.N. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity 15, 691–701 (2001).
Roumier, A. et al. The membrane-microfilament linker ezrin is involved in the formation of the immunological synapse and in T cell activation. Immunity 15, 715–728 (2001).
Bretscher, A., Edwards, K. & Fehon, R.G. ERM proteins and merlin: integrators at the cell cortex. Nat. Rev. Mol. Cell Biol. 3, 586–599 (2002).
Gautreau, A., Louvard, D. & Arpin, M. ERM proteins and NF2 tumor suppressor: the Yin and Yang of cortical actin organization and cell growth signaling. Curr. Opin. Cell Biol. 14, 104–109 (2002).
Ridley, A.J. Rho family proteins: coordinating cell responses. Trends Cell Biol. 11, 471–477 (2001).
Etienne-Manneville, S. & Hall, A. Rho GTPases in cell biology. Nature 420, 629–635 (2002).
Cantrell, D.A. GTPases and T cell activation. Immunol. Rev. 192, 122–130 (2003).
Shcherbina, A., Bretscher, A., Kenney, D.M. & Remold-O'Donnell, E. Moesin, the major ERM protein of lymphocytes and platelets, differs from ezrin in its insensitivity to calpain. FEBS Lett. 443, 31–36 (1999).
Pietromonaco, S.F., Simons, P.C., Altman, A. & Elias, L. Protein kinase C-θ phosphorylation of moesin in the actin-binding sequence. J. Biol. Chem. 273, 7594–7603 (1998).
Nobes, C.D., Hawkins, P., Stephens, L. & Hall, A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J. Cell Sci. 108, 225–233 (1995).
Matsui, T., Yonemura, S. & Tsukita, S. Activation of ERM proteins in vivo by Rho involves phosphatidyl-inositol 4-phosphate 5-kinase and not ROCK kinases. Curr. Biol. 9, 1259–1262 (1999).
Salazar-Fontana, L.I., Barr, V., Samelson, L.E. & Bierer, B.E. CD28 engagement promotes actin polymerization through the activation of the small Rho GTPase Cdc42 in human T cells. J. Immunol. 171, 2225–2232 (2003).
Aspenstrom, P. Effectors for the Rho GTPases. Curr. Opin. Cell Biol. 11, 95–102 (1999).
Lamarche, N. et al. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65PAK and the JNK/SAPK MAP kinase cascade. Cell 87, 519–529 (1996).
Gomez, M., Kioussis, D. & Cantrell, D.A. The GTPase Rac-1 controls cell fate in the thymus by diverting thymocytes from positive to negative selection. Immunity 15, 703–713 (2001).
Turner, M. & Billadeau, D.D. VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486 (2002).
Tybulewicz, V.L., Ardouin, L., Prisco, A. & Reynolds, L.F. Vav1: a key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 (2003).
Reynolds, L.F. et al. Vav1 transduces T cell receptor signals to the activation of phospholipase C-γ1 via phosphoinositide 3-kinase-dependent and -independent pathways. J. Exp. Med. 195, 1103–1114 (2002).
Fischer, K.-D. et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr. Biol. 8, 554–562 (1998).
Holsinger, L.J. et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 8, 563–572 (1998).
Costello, P.S. et al. The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kB pathways. Proc. Natl. Acad. Sci. USA 96, 3035–3040 (1999).
Brown, M.J. et al. Chemokine stimulation of human peripheral blood T lymphocytes induces rapid dephosphorylation of ERM proteins, which facilitates loss of microvilli and polarization. Blood 102, 3890–3899 (2003).
Crepaldi, T., Gautreau, A., Comoglio, P.M., Louvard, D. & Arpin, M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J. Cell Biol. 138, 423–434 (1997).
Amieva, M.R., Litman, P., Huang, L., Ichimaru, E. & Furthmayr, H. Disruption of dynamic cell surface architecture of NIH3T3 fibroblasts by the N-terminal domains of moesin and ezrin: in vivo imaging with GFP fusion proteins. J. Cell Sci. 112, 111–125 (1999).
Yoshinaga-Ohara, N., Takahashi, A., Uchiyama, T. & Sasada, M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp. Cell Res. 278, 112–122 (2002).
Kondo, T. et al. ERM (ezrin/radixin/moesin)-based molecular mechanism of microvillar breakdown at an early stage of apoptosis. J. Cell Biol. 139, 749–758 (1997).
Stefanova, I., Dorfman, J.R. & Germain, R.N. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature 420, 429–434 (2002).
Krawczyk, C. et al. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity 16, 331–343 (2002).
Ardouin, L. et al. Vav1 transduces TCR signals required for LFA-1 function and cell polarization at the immunological synapse. Eur. J. Immunol. 33, 790–797 (2003).
Qi, S.Y., Groves, J.T. & Chakraborty, A.K. Synaptic pattern formation during cellular recognition. Proc. Natl. Acad. Sci. USA 98, 6548–6553 (2001).
Lee, S.J., Hori, Y., Groves, J.T., Dustin, M.L. & Chakraborty, A.K. The synapse assembly model. Trends Immunol. 23, 500–502 (2002).
Wülfing, C. et al. Costimulation and endogenous MHC ligands contribute to T cell recognition. Nat. Immunol. 3, 42–47 (2002).
Turner, M. et al. A requirement for the Rho-family GTP exchange factor Vav in positive and negative selection of thymocytes. Immunity 7, 451–460 (1997).
Acknowledgements
We thank J.K. Burkhardt, N. Hotchin and A. Hall for providing us with constructs; and S. Shaw, C. Randriamampita and E. Donnadieu for critical reading of the manuscript and suggestions. Supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, Ligue Nationale contre le Cancer, Région Ile-de-France and Association Claude Bernard (M.S.).
*Note: In the version of this article originally published online, the labeling of the last two lanes in Figure 2a was incorrect. They should read "GFP-Rac1L61" and "GFP-Cdc42L61." This error has been corrected for the HTML and print versions of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Faure, S., Salazar-Fontana, L., Semichon, M. et al. ERM proteins regulate cytoskeleton relaxation promoting T cell–APC conjugation. Nat Immunol 5, 272–279 (2004). https://doi.org/10.1038/ni1039
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1039
This article is cited by
-
Role of Rho GTPases in inflammatory bowel disease
Cell Death Discovery (2023)
-
RHO GTPases: from new partners to complex immune syndromes
Nature Reviews Immunology (2021)
-
Alpha-mangostin dephosphorylates ERM to induce adhesion and decrease surface stiffness in KG-1 cells
Human Cell (2021)
-
Flow-cytometric microglial sorting coupled with quantitative proteomics identifies moesin as a highly-abundant microglial protein with relevance to Alzheimer’s disease
Molecular Neurodegeneration (2020)
-
Interaction of calcium binding protein S100A16 with myosin-9 promotes cytoskeleton reorganization in renal tubulointerstitial fibrosis
Cell Death & Disease (2020)