Abstract
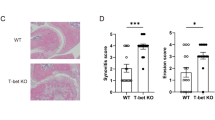
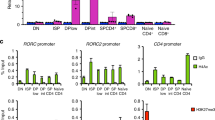
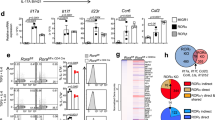
Overactive responses by interleukin 17 (IL-17)-producing helper T cells (TH17 cells) are tightly linked to the development of autoimmunity, yet the factors that negatively regulate the differentiation of this lineage remain unknown. Here we report that the transcription factor T-bet suppressed development of the TH17 cell lineage by inhibiting transcription of Rorc (which encodes the transcription factor RORγt). T-bet interacted with the transcription factor Runx1, and this interaction blocked Runx1-mediated transactivation of Rorc. T-bet Tyr304 was required for formation of the T-bet–Runx1 complex, for blockade of Runx1 activity and for inhibition of the TH17 differentiation program. Our data reinforce the idea of master regulators that shape immune responses by simultaneously activating one genetic program while silencing the activity of competing regulators in a common progenitor cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mosmann, T.R., Cherwinski, H., Bond, M.W., Giedlin, M.A. & Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136, 2348–2357 (1986).
Aggarwal, S., Ghilardi, N., Xie, M.H., de Sauvage, F.J. & Gurney, A.L. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278, 1910–1914 (2003).
Harrington, L.E. et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132 (2005).
Langrish, C.L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Mangan, P.R. et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature 441, 231–234 (2006).
Park, H. et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141 (2005).
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Liang, S.C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279 (2006).
Veldhoen, M., Hocking, R.J., Atkins, C.J., Locksley, R.M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Zhou, L. et al. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 (2007).
Ivanov, I.I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006).
Korn, T. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007).
McGeachy, M.J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Djuretic, I.M. et al. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Nat. Immunol. 8, 145–153 (2007).
Hwang, E.S., Szabo, S.J., Schwartzberg, P.L. & Glimcher, L.H. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science 307, 430–433 (2005).
Burrell, B.E., Csencsits, K., Lu, G., Grabauskiene, S. & Bishop, D.K. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J. Immunol. 181, 3906–3914 (2008).
Rangachari, M. et al. T-bet negatively regulates autoimmune myocarditis by suppressing local production of interleukin 17. J. Exp. Med. 203, 2009–2019 (2006).
Yuan, X. et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J. Exp. Med. 205, 3133–3144 (2008).
Guo, S., Cobb, D. & Smeltz, R.B. T-bet inhibits the in vivo differentiation of parasite-specific CD4+ Th17 cells in a T cell-intrinsic manner. J. Immunol. 182, 6179–6186 (2009).
Hegazy, A.N. et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3+T-bet+ cell subset with combined Th2 and Th1 cell functions. Immunity 32, 116–128 (2010).
Lee, Y.K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Szabo, S.J. et al. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100, 655–669 (2000).
Bettelli, E. et al. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J. Exp. Med. 200, 79–87 (2004).
McGeachy, M.J. et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
Liu, X. et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat. Med. 16, 191–197 (2010).
Garrett, W.S. et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 131, 33–45 (2007).
Hwang, E.S., Hong, J.H. & Glimcher, L.H. IL-2 production in developing Th1 cells is regulated by heterodimerization of Re1A and T-bet and requires T-bet serine residue 508. J. Exp. Med. 202, 1289–1300 (2005).
Abromson-Leeman, S., Bronson, R.T. & Dorf, M.E. Encephalitogenic T cells that stably express both T-bet and RORγt consistently produce IFNγ but have a spectrum of IL-17 profiles. J. Neuroimmunol. 215, 10–24 (2009).
Mehta, D.S., Wurster, A.L., Weinmann, A.S. & Grusby, M.J. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc. Natl. Acad. Sci. USA 102, 2016–2021 (2005).
Zhang, F., Meng, G. & Strober, W. Interactions among the transcription factors Runx1, RORγt and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 9, 1297–1306 (2008).
Brustle, A. et al. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8, 958–966 (2007).
Schraml, B.U. et al. The AP-1 transcription factor Batf controls TH17 differentiation. Nature 460, 405–409 (2009).
Durant, L. et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity 32, 605–615 (2010).
Jenner, R.G. et al. The transcription factors T-bet and GATA-3 control alternative pathways of T-cell differentiation through a shared set of target genes. Proc. Natl. Acad. Sci. USA 106, 17876–17881 (2009).
Gocke, A.R. et al. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J. Immunol. 178, 1341–1348 (2007).
Yang, Y. et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206, 1549–1564 (2009).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Veldhoen, M. et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 (2008).
Veldhoen, M., Hirota, K., Christensen, J., O′Garra, A. & Stockinger, B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206, 43–49 (2009).
Harris, T.J. et al. Cutting edge: An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 (2007).
Liu, X., Lee, Y.S., Yu, C.R. & Egwuagu, C.E. Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 180, 6070–6076 (2008).
Mathur, A.N. et al. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 178, 4901–4907 (2007).
Mukasa, R. et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity 32, 616–627 (2010).
Bromberg, J.F. et al. Stat3 as an oncogene. Cell 98, 295–303 (1999).
Cawley, S. et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116, 499–509 (2004).
Jones, D.C. et al. Regulation of adult bone mass by the zinc finger adapter protein Schnurri-3. Science 312, 1223–1227 (2006).
Acknowledgements
We thank D. Kozoriz for help in cell sorting; W. Strober (National Institute of Allergy and Infectious Diseases) for retroviral plasmids encoding Runx1 and dominant negative Runx1; S.L. Reiner (University of Pennsylvania) for mice with loxP-flanked Tbx21 alleles; C.B. Wilson (The Bill and Melinda Gates Foundation) for mice with expression of Cre recombinase driven by the Cd4 promoter; and A.-H. Lee, T. Staton-Winslow, M. Greenblatt and M. Wein for critical review of the manuscript. Supported by the US National Institutes of Health (P01 NS038037), the Ragon Institute of MIT, MGH and Harvard (L.H.G.), the National Cancer Research Center (R15-2006-020 to E.S.H.) and the Cancer Research Institute (V.L.).
Author information
Authors and Affiliations
Contributions
V.L. designed and did experiments and prepared the manuscript; X.C. did ChIP assays; J.-H.S. did DNA-precipitation and coimmunoprecipitation assays; E.-S.H. created T-bet mutant retroviral constructs; E.J. did doxycycline transgenic T cell experiments; M.O. generated IL-23R.GFP mice; V.K.K. contributed to discussions and manuscript preparation; A.N.B. provided technical assistance; and L.H.G. supervised the research, designed experiments and participated in preparing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
L.H.G. is on the Board of Directors and holds equity in Bristol Myers Squibb.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Supplementary Tables 1–2 (PDF 1914 kb)
Rights and permissions
About this article
Cite this article
Lazarevic, V., Chen, X., Shim, JH. et al. T-bet represses TH17 differentiation by preventing Runx1-mediated activation of the gene encoding RORγt. Nat Immunol 12, 96–104 (2011). https://doi.org/10.1038/ni.1969
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1969
This article is cited by
-
Pumpkin seeds (Cucurbita pepo subsp. ovifera) decoction promotes Trichinella spiralis expulsion during intestinal phase via “Weep and Sweep” mechanism
Scientific Reports (2024)
-
The role of transcription factors in shaping regulatory T cell identity
Nature Reviews Immunology (2023)
-
IFNγ-induction of TH1-like regulatory T cells controls antiviral responses
Nature Immunology (2023)
-
An IL-17-EGFR-TRAF4 axis contributes to the alleviation of lung inflammation in severe influenza
Communications Biology (2023)
-
Markers and makers of NKT17 cells
Experimental & Molecular Medicine (2023)