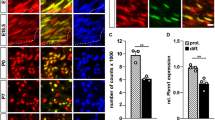
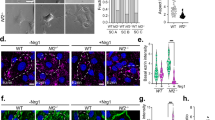
Abstract
Neurofibromatosis type 2 is an autosomal dominant disorder characterized by tumors, predominantly schwannomas, in the nervous system. It is caused by mutations in the gene NF2, encoding the growth regulator schwannomin (also known as merlin). Mutations occur throughout the 17-exon gene, with most resulting in protein truncation and undetectable amounts of schwannomin protein. Pathogenic mutations that result in production of defective schwannomin include in-frame deletions of exon 2 and three independent missense mutations within this same exon. Mice with conditional deletion of exon 2 in Schwann cells develop schwannomas, which confirms the crucial nature of exon 2 for growth control. Here we report that the molecular adaptor paxillin binds directly to schwannomin at residues 50–70, which are encoded by exon 2. This interaction mediates the membrane localization of schwannomin to the plasma membrane, where it associates with β1 integrin and erbB2. It defines a pathogenic mechanism for the development of NF2 in humans with mutations in exon 2 of NF2.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rouleau, G.A. et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature 363, 515–521 (1993).
Trofatter, J.A. et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumour suppressor. Cell 72, 791–800 (1993); erratum Cell 75, 826 (1993).
Jacoby, L.B., MacCollin, M., Barone R., Ramesh, V. & Gusella J.F. Frequency and distribution of NF2 mutations in schwannomas. Genes Chromosom. Cancer 17, 45–55 (1996).
Bourn, D., Carter, S., Gareth, D., Evans, R. & Strachan, T. Germline mutations in the neurofibromatosis type 2 tumour suppressor gene. Hum. Mol. Genet. 3, 813–816 (1994).
Scoles, D.R. Baser, M.E. & Pulst, S.M. A missense mutation in the neurofibromatosis 2 gene occurs in patients with mild and severe phenotypes. Neurology 47, 544–546 (1996).
Bianchi, A.B. et al. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nature Genet. 6, 185–192 (1994).
Welling, D.B. et al. Mutational spectrum in the neurofibromatosis type 2 gene in sporadic and familial schwannomas. Hum. Genet. 98, 189–193 (1996).
Giovannini, M. et al. Conditional biallelic Nf2 mutation in the mouse promotes manifestations of human neurofibromatosis type 2. Genes Dev. 14, 1617–1630 (2000).
Deguen, B. et al. Impaired interaction of naturally occurring mutant NF2 protein with actin-based cytoskeleton and membrane. Hum. Mol. Genet. 7, 217–226 (1998).
Heiska, L. et al. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 273, 21893–21900 (1998).
Sainio, M. et al. Neurofibromatosis 2 tumour suppressor protein colocalizes with ezrin and CD44 and associates with actin-containing cytoskeleton. J. Cell Sci. 110, 2249–2260 (1997).
Xing, B., Jedsadayanmata, A. & Lam, S.C. Localization of an integrin binding site to the C terminus of talin. J. Biol. Chem. 276, 44373–44378 (2001).
Obremski, V.J., Hall, A.M. & Fernandez-Valle, C. Schwannomin, the neurofibromatosis type 2 gene product, and β1 integrin associate in isolated and differentiating Schwann cell. J. Neurobiol. 37, 487–501 (1998).
Scoles, D.R. et al. Neurofibromatosis 2 tumour suppressor schwannomin interacts with βII spectrin. Nature Genet. 18, 354–359 (1998).
Gonzalez-Agosti, C., Wiederhold, T., Herndon, M.E., Gusella, J. & Ramesh, V. Interdomain interaction of schwannomin isoforms and its influence on intermolecular binding to NHE-RF. J. Biol. Chem. 274, 34438–34442 (1999).
Gronholm, M. et al. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumour suppressor protein schwannomin and the ERM protein ezrin. J. Cell Sci. 112, 895–904. (1999).
Goutebroze, L., Brault, E., Muchardt, C., Camonis, J. & Thomas, G. Cloning and characterization of SCHIP-1, a novel protein interacting specifically with spliced isoforms and naturally occurring mutant NF2 proteins. Mol. Cell. Biol. 20, 1699–1712 (2000).
Jannatipour, J. et al. Schwannomin isoform-1 interacts with syntenin via PDZ domains. J. Biol. Chem. 276, 33093–33100 (2001).
Pykett, M.J., Murphy, M., Harnish, P.R. & George, D.L. The neurofibromatosis 2 (NF2) tumour suppressor gene encodes multiple alternatively spliced transcripts. Hum. Mol. Genet. 3, 559–564 (1994).
Turner, C.E. Paxillin interactions. J. Cell Sci. 113, 4139–4140 (2000).
Turner, C.E. & Miller, J.T. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J. Cell Sci. 107, 1583–1591 (1994).
Brown, M.C., Perrotta, J.A. & Turner, C.E. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol. Biol. Cell. 9, 1803–1816 (1998).
Norman, J.C. et al. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J. Cell Biol. 143, 1981–1995 (1998).
Turner, C.E. et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J. Cell Biol. 145, 851–863 (1999).
Shaw, R.J. et al. Nf2 tumour suppressor, merlin, functions in Rac-dependent signaling. Dev. Cell. 1, 63–72 (2001).
Xiao, G.-H., Beeser, A., Chernoff, J. & Testa, J.R. p21-activated kinase links Rac/Cdc42 signaling to merlin. J. Biol. Chem. 277, 883–886 (2002).
Eldridge, C.F., Bunge, M.B., Bunge, R.P. & Wood, P.M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 105, 1023–1034 (1987).
Fernandez-Valle, C., Fregien, N., Wood, P.M. & Bunge, M.B. Expression of the protein zero myelin gene in axon-related Schwann cells is linked to basal lamina formation. Development 119, 867–880 (1993).
Fields, R.D. Effects of ion channel activity on development of dorsal root ganglion neurons. J. Neurobiol. 37, 158–170 (1998).
Bunge, R.P. & Fernandez-Valle, C. in Neuralglial Cells (eds Kettenmann, H. & Ransom, B.) 44–57 (Oxford Univ. Press, Oxford, 1995).
Marchionni, M.A. et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature 362, 312–318 (1993).
Fernandez-Valle, C., Gwynn, L., Wood, P.M., Carbonetto, S. & Bunge, M.B. Anti-β1 integrin antibody inhibits Schwann cell myelination. J. Neurobiol. 25, 1207–1226 (1994).
Morrissey, T.K., Levi, A.D., Nuijens, A., Sliwkowski, M.X. & Bunge, R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl Acad. Sci. USA 92, 1431–1435 (1995).
Vartanian, T., Goodearl, A., Viehover, A. & Fischbach, G. Axonal neuregulin signals cells of the oligodendrocyte lineage through activation of HER4 and Schwann cells through HER2 and HER3. J. Cell Biol. 137, 211–220 (1997).
Chen, L.M., Bailey, D. & Fernandez-Valle, C. Association of β1 integrin with focal adhesion kinase and paxillin in differentiating Schwann cells. J. Neurosci. 20, 3776–3784 (2000).
Fernandez-Valle, C., Gorman, D., Gomez, A.M. & Bunge, M.B. Actin plays a role in both changes in cell shape and gene expression associated with Schwann cell myelination. J. Neurosci. 17, 241–250 (1997).
Plopper, G. & Ingber, D.E. Rapid induction and isolation of focal adhesion complexes. Biochem. Biophys. Res. Commun. 193, 571–578 (1993).
Schmucker, B., Ballhausen, W.G. & Kressel, M. Subcellular localization and expression pattern of the neurofibromatosis type 2 protein merlin/schwannomin. Eur. J. Cell Biol. 72, 46–53 (1997).
Kangs, B.S., Cooper, D.R., Devedjiev, Y., Derewenda, U. & Derewenda, Z.S. The structure of the FERM domain of merlin, the neurofibromatosis type 2 gene product. Acta Crystallogr. D. 58, 381–391 (2002).
Vadlamudi, R., Adam, L., Talukder, A., Mendelsohn, J. & Kumar, R. Serine phosphorylation of paxillin by heregulin-β1: role of p38 mitogen activated protein kinase. Oncogene 18, 7253–7264 (1999).
Edwards, S.D. & Keep, N.H. The 2.7 Å crystal structure of the activated ferm domain of moesin: an analysis of structural changes on activation. Biochemistry 40, 7061–7068 (2001).
Matsui, T. et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J. Cell Biol. 140, 647–657 (1998).
Turunen, O., Sainio, M., Jaaskelainen, J., Carpen, O. & Vaheri, A. Structure-function relationships in the ezrin family and the effect of tumour-associated point mutations in neurofibromatosis 2 protein. Biochim. Biophys. Acta 1387, 1–16 (1998).
Stokowski, R.A. & Cox D.R. Functional analysis of the neurofibromatosis type 2 protein by means of disease-causing point mutations. Am. J. Hum. Genet. 66, 873–891 (2000).
West, K.A. et al. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL). J. Cell Biol. 154, 161–167 (2001).
Nikolopoulos, S.N & Turner, C.E. Molecular dissection of actopaxin-integrin-linked kinase-paxillin interactions and their role in subcellular localization. J. Biol. Chem. 277, 1568–1575 (2002).
Turner, C.E., West, K.A. & Brown, M.C. Paxillin-ARF GAP signaling and the cytoskeleton. Curr. Opin. Cell Biol. 13, 593–599 (2001).
Scherer, S.S. & Gutmann, D.H. Expression of the neurofibromatosis 2 tumour suppressor gene product, schwannomin, in Schwann cells. J. Neurosci. Res. 46, 595–605 (1996).
Morrison, H. et al. The NF2 tumour suppressor gene product, merlin, mediates contact inhibition of growth through interactions with CD44. Genes Dev. 15, 968–980 (2001).
Scoles, D.R. et al. The neurofibromatosis 2 tumour suppressor protein interacts with hepatocyte growth factor-regulated tyrosine kinase substrate. Hum. Mol. Genet. 9, 1567–1574 (2000).
Acknowledgements
We thank C. Turner for paxillin cDNA; S. Pulst and L. Sherman for discussions; and D. Bailey and E. Rodriguez for technical assistance. This work was supported by a US Public Health Service grant (to C.F.-V.), a National Neurofibromatosis Foundation Young Investigator Award (to J.R.) and the State of Florida.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Fernandez-Valle, C., Tang, Y., Ricard, J. et al. Paxillin binds schwannomin and regulates its density-dependent localization and effect on cell morphology. Nat Genet 31, 354–362 (2002). https://doi.org/10.1038/ng930
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng930
This article is cited by
-
The genetic landscape and possible therapeutics of neurofibromatosis type 2
Cancer Cell International (2023)
-
Lipid binding promotes the open conformation and tumor-suppressive activity of neurofibromin 2
Nature Communications (2018)
-
Role of Merlin/NF2 inactivation in tumor biology
Oncogene (2016)
-
Oncogenic role of Merlin/NF2 in glioblastoma
Oncogene (2015)
-
DNA copy gains of tumor-related genes in vestibular schwannoma
European Archives of Oto-Rhino-Laryngology (2013)