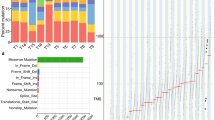
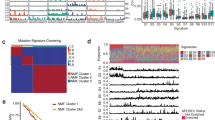
Abstract
Esophageal squamous cell carcinoma (ESCC) is prevalent worldwide and particularly common in certain regions of Asia. Here we report the whole-exome or targeted deep sequencing of 139 paired ESCC cases, and analysis of somatic copy number variations (SCNV) of over 180 ESCCs. We identified previously uncharacterized mutated genes such as FAT1, FAT2, ZNF750 and KMT2D, in addition to those already known (TP53, PIK3CA and NOTCH1). Further SCNV evaluation, immunohistochemistry and biological analysis suggested their functional relevance in ESCC. Notably, RTK-MAPK-PI3K pathways, cell cycle and epigenetic regulation are frequently dysregulated by multiple molecular mechanisms in this cancer. Our approaches also uncovered many druggable candidates, and XPO1 was further explored as a therapeutic target because it showed both gene mutation and protein overexpression. Our integrated study unmasks a number of novel genetic lesions in ESCC and provides an important molecular foundation for understanding esophageal tumors and developing therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Zhao, P., Dai, M., Chen, W. & Li, N. Cancer trends in China. Jpn. J. Clin. Oncol. 40, 281–285 (2010).
Pennathur, A., Gibson, M.K., Jobe, B.A. & Luketich, J.D. Oesophageal carcinoma. Lancet 381, 400–412 (2013).
Yang, Y.L. et al. Amplification of PRKCI, located in 3q26, is associated with lymph node metastasis in esophageal squamous cell carcinoma. Genes Chromosom. Cancer 47, 127–136 (2008).
Bass, A.J. et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 41, 1238–1242 (2009).
Luo, M.L. et al. Amplification and overexpression of CTTN (EMS1) contribute to the metastasis of esophageal squamous cell carcinoma by promoting cell migration and anoikis resistance. Cancer Res. 66, 11690–11699 (2006).
Hu, N. et al. Genomic characterization of esophageal squamous cell carcinoma from a high-risk population in China. Cancer Res. 69, 5908–5917 (2009).
Shigaki, H. et al. PIK3CA mutation is associated with a favorable prognosis among patients with curatively resected esophageal squamous cell carcinoma. Clin. Cancer Res. 19, 2451–2459 (2013).
Agrawal, N. et al. Comparative genomic analysis of esophageal adenocarcinoma and squamous cell carcinoma. Cancer Discov. 2, 899–905 (2012).
Shi, Z.Z. et al. Genomic alterations with impact on survival in esophageal squamous cell carcinoma identified by array comparative genomic hybridization. Genes Chromosom. Cancer 50, 518–526 (2011).
Hirasaki, S. et al. BAC clones related to prognosis in patients with esophageal squamous carcinoma: an array comparative genomic hybridization study. Oncologist 12, 406–417 (2007).
Hu, N. et al. Genome wide analysis of DNA copy number neutral loss of heterozygosity (CNNLOH) and its relation to gene expression in esophageal squamous cell carcinoma. BMC Genomics 11, 576 (2010).
Beroukhim, R. et al. The landscape of somatic copy-number alteration across human cancers. Nature 463, 899–905 (2010).
Lawrence, M.S. et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 499, 214–218 (2013).
Shah, S.P. et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399 (2012).
Burns, M.B., Temiz, N.A. & Harris, R.S. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat. Genet. 45, 977–983 (2013).
Burns, M.B. et al. APOBEC3B is an enzymatic source of mutation in breast cancer. Nature 494, 366–370 (2013).
Roberts, S.A. et al. An APOBEC cytidine deaminase mutagenesis pattern is widespread in human cancers. Nat. Genet. 45, 970–976 (2013).
Shi, Z.Z. et al. Consistent and differential genetic aberrations between esophageal dysplasia and squamous cell carcinoma detected by array comparative genomic hybridization. Clin. Cancer Res. 19, 5867–5878 (2013).
Guagnano, V. et al. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov. 2, 1118–1133 (2012).
Lin, D.C., Du, X.L. & Wang, M.R. Protein alterations in ESCC and clinical implications: a review. Dis. Esophagus 22, 9–20 (2009).
Zhang, Y. et al. Reciprocal activation between PLK1 and Stat3 contributes to survival and proliferation of esophageal cancer cells. Gastroenterology 142, 521–530 (2012).
Liu, K. et al. Sox2 cooperates with inflammation-mediated Stat3 activation in the malignant transformation of foregut basal progenitor cells. Cell Stem Cell 12, 304–315 (2013).
Cohen, I. et al. ZNF750 is expressed in differentiated keratinocytes and regulates epidermal late differentiation genes. PLoS ONE 7, e42628 (2012).
Sen, G.L. et al. ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev. Cell 22, 669–677 (2012).
Yu, X. et al. Differentiation-associated genes regulated by TPA-induced c-Jun expression via a PKC/JNK pathway in KYSE450 cells. Biochem. Biophys. Res. Commun. 342, 286–292 (2006).
Chen, H. et al. S100A14 is a novel modulator of terminal differentiation of esophageal squamous cell carcinoma. Mol. Cancer Res. 11, 1542–1553 (2013).
Morris, L.G. et al. Recurrent somatic mutation of FAT1 in multiple human cancers leads to aberrant Wnt activation. Nat. Genet. 45, 253–261 (2013).
Dikshit, B. et al. FAT1 acts as an upstream regulator of oncogenic and inflammatory pathways, via PDCD4, in glioma cells. Oncogene 32, 3798–3808 (2013).
de Bock, C.E. et al. The Fat1 cadherin is overexpressed and an independent prognostic factor for survival in paired diagnosis-relapse samples of precursor B-cell acute lymphoblastic leukemia. Leukemia 26, 918–926 (2012).
Turner, J.G., Dawson, J. & Sullivan, D.M. Nuclear export of proteins and drug resistance in cancer. Biochem. Pharmacol. 83, 1021–1032 (2012).
Landau, D.A. et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152, 714–726 (2013).
Paraskeva, E. et al. CRM1-mediated recycling of snurportin 1 to the cytoplasm. J. Cell Biol. 145, 255–264 (1999).
Monecke, T. et al. Crystal structure of the nuclear export receptor CRM1 in complex with Snurportin1 and RanGTP. Science 324, 1087–1091 (2009).
Ranganathan, P. et al. Preclinical activity of a novel CRM1 inhibitor in acute myeloid leukemia. Blood 120, 1765–1773 (2012).
Etchin, J. et al. KPT-330 inhibitor of CRM1 (XPO1)-mediated nuclear export has selective anti-leukaemic activity in preclinical models of T-cell acute lymphoblastic leukaemia and acute myeloid leukaemia. Br. J. Haematol. 161, 117–127 (2013).
Etchin, J. et al. Antileukemic activity of nuclear export inhibitors that spare normal hematopoietic cells. Leukemia 27, 66–74 (2013).
Chen, L., Fischle, W., Verdin, E. & Greene, W.C. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science 293, 1653–1657 (2001).
Kanezaki, R. et al. Transcription factor BACH1 is recruited to the nucleus by its novel alternative spliced isoform. J. Biol. Chem. 276, 7278–7284 (2001).
Kim, J.Y. & Casaccia, P. HDAC1 in axonal degeneration: a matter of subcellular localization. Cell Cycle 9, 3680–3684 (2010).
Lapalombella, R. et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood 120, 4621–4634 (2012).
Schmidt, J. et al. Genome-wide studies in multiple myeloma identify XPO1/CRM1 as a critical target validated using the selective nuclear export inhibitor KPT-276. Leukemia 27, 2357–2365 (2013).
Walker, C.J. et al. Preclinical and clinical efficacy of XPO1/CRM1 inhibition by the karyopherin inhibitor KPT-330 in Ph+ leukemias. Blood 122, 3034–3044 (2013).
Tai, Y.T. et al. CRM1 inhibition induces tumor cell cytotoxicity and impairs osteoclastogenesis in multiple myeloma: molecular mechanisms and therapeutic implications. Leukemia 28, 155–165 (2014).
Huang, W.Y. et al. Prognostic value of CRM1 in pancreas cancer. Clin. Invest. Med. 32, E315 (2009).
Kojima, K. et al. Prognostic impact and targeting of CRM1 in acute myeloid leukemia. Blood 121, 4166–4174 (2013).
Noske, A. et al. Expression of the nuclear export protein chromosomal region maintenance/exportin 1/Xpo1 is a prognostic factor in human ovarian cancer. Cancer 112, 1733–1743 (2008).
Shen, A. et al. Expression of CRM1 in human gliomas and its significance in p27 expression and clinical prognosis. Neurosurgery 65, 153–159, discussion 159–160 (2009).
Wei, Q. et al. EGFR, HER2 and HER3 expression in esophageal primary tumours and corresponding metastases. Int. J. Oncol. 31, 493–499 (2007).
Sato-Kuwabara, Y., Neves, J.I., Fregnani, J.H., Sallum, R.A. & Soares, F.A. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using fluorescence in situ hybridization (FISH) and immunohistochemistry. BMC Cancer 9, 6 (2009).
Boone, J. et al. mTOR in squamous cell carcinoma of the oesophagus: a potential target for molecular therapy? J. Clin. Pathol. 61, 909–913 (2008).
Akagi, I. et al. Overexpression of PIK3CA is associated with lymph node metastasis in esophageal squamous cell carcinoma. Int. J. Oncol. 34, 767–775 (2009).
Garnett, M.J. et al. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature 483, 570–575 (2012).
Somaiah, N. & Simon, G.R. Molecular targeted agents and biologic therapies for lung cancer. J. Thorac. Oncol. 6, S1758–S1785 (2011).
Su, H. et al. Global gene expression profiling and validation in esophageal squamous cell carcinoma and its association with clinical phenotypes. Clin. Cancer Res. 17, 2955–2966 (2011).
Zhu, Y.H. et al. Downregulation of the novel tumor suppressor DIRAS1 predicts poor prognosis in esophageal squamous cell carcinoma. Cancer Res. 73, 2298–2309 (2013).
Lee, D.H. et al. Synergistic effect of low-dose cucurbitacin B and low-dose methotrexate for treatment of human osteosarcoma. Cancer Lett. 306, 161–170 (2011).
Sato, Y. et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat. Genet. 45, 860–867 (2013).
Sakaguchi, H. et al. Exome sequencing identifies secondary mutations of SETBP1 and JAK3 in juvenile myelomonocytic leukemia. Nat. Genet. 45, 937–941 (2013).
Yoshida, K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011).
Lin, D.C. et al. Genomic and functional characterizations of phosphodiesterase subtype 4D in human cancers. Proc. Natl. Acad. Sci. USA 110, 6109–6114 (2013).
Nannya, Y. et al. A robust algorithm for copy number detection using high-density oligonucleotide single nucleotide polymorphism genotyping arrays. Cancer Res. 65, 6071–6079 (2005).
Yamamoto, G. et al. Highly sensitive method for genomewide detection of allelic composition in nonpaired, primary tumor specimens by use of affymetrix single-nucleotide-polymorphism genotyping microarrays. Am. J. Hum. Genet. 81, 114–126 (2007).
Eden, E., Navon, R., Steinfeld, I., Lipson, D. & Yakhini, Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics 10, 48 (2009).
Zhang, B., Kirov, S. & Snoddy, J. WebGestalt: an integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res. 33, W741–W748 (2005).
Wang, J., Duncan, D., Shi, Z. & Zhang, B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 41, W77–W83 (2013).
Acknowledgements
We thank P. Tan and Z. Zang for generously sharing related facilities. We also thank B. Koegler and S. Koegler for their generous support. This work is supported by National High-Tech R&D Program of China 2012AA02A503 and 2012AA02A209 (M.-R.W.), National Natural Science Foundation of China 81330052 (M.-R.W.), US National Institutes of Health grant R01CA026038-35 (H.P.K.), National Research Foundation Singapore and the Singapore Ministry of Education under the Research Centers of Excellence initiative (H.P.K.), and Singapore Ministry of Health's National Medical Research Council under its Singapore Translational Research (STaR) Investigator Award to H.P.K.
Author information
Authors and Affiliations
Contributions
D.-C.L., M.-R.W. and H.P.K. designed the study and wrote the manuscript. D.-C.L., J.-J.H., Y.N., Y.S., Y.O., X.M., L.X., A.M.V., L.-W.D. and M.G. performed experiments. D.Y, J.-J.H., Z.-Z.S., L.S., Y.-Y.J., W.-Y.G., T.G., Y.Z. and X.X. coordinated sample collection and processing. O.K. and S.S. provided KPT-330 and structurally analyzed XPO1 point mutation. D.-C.L., J.-J.H., Y.N., S.O., M.-R.W. and H.P.K. analyzed and discussed the data. Y.N., H.Y., L.-Z.L., Y.S. and Y.O. performed bioinformatical analysis.
Corresponding authors
Ethics declarations
Competing interests
O.K. and S.S. are employees of Karyopharm Therapeutics Incorporated. The remaining authors declare no conflict of interest.
Integrated supplementary information
Supplementary Figure 1 Somatic mutation frequencies detected in exomes from ESCC and other human cancers.
Each dot represents an examined case (tumor-germline pair), and tumor types are ordered by their median somatic mutation frequencies (indicated by the black dash line). The number beneath X-axis indicates the number of examined cases of each cancer. Except ESCC, all of the other cancers were sequenced and published elsewhere, and their data were re-analyzed by Lawrence et al. CLL, chronic lymphocytic leukemia; MM, multiple myeloma; GBM, glioblastoma multiforme; DLBCL, diffuse large B-cell lymphoma; HNSCC, head and neck squamous cell carcinoma; EAC, esophageal adenocarcinoma; Lung AC, lung adenocarcinoma; Lung SCC, lung squamous cell carcinoma.
Supplementary Figure 2 Intratumoral clonality analysis.
Intratumoral clonality plots of four representative ESCC cases from Discovery Cohort. ESCC-D13, D15, D17 tumors were developed bi-clonaly whereas ESCC-D14 tumor showed multi-clonal structure. Variant allele frequency was calculated with copy-number neutral variants, which was further used to plot intratumoral clonal architectures.
Supplementary Figure 3 Mutation types and signatures in ESCC.
(a) The occurrence of the six types of base-substitution mutations observed in ESCC. (b) The occurrence of cytosine mutations at each group of trinucleotide, which shows biases toward mutations of TCN motifs. (c) Genes mutated in over 5% (≥ 7 cases) of the entire cohort.
Supplementary Figure 4 Down-regulation of FBXW7 protein expression in ESCC.
Representative FBXW7 IHC results of one ESCC case from Additional Cohort (Upper panel) and one ESCC case from Frequency Cohort with FBXW7 mutation. Scale bars, 200 μm.
Supplementary Figure 5 Identification of ZNF750 as a novel recessive cancer gene in ESCC.
(a) Box plot showing relative ZNF750 mRNA levels in 947 human cancer cell lines across 21 cancer types analyzed from CCLE database (see URL for the description of Box Plot). Number in the parentheses indicates the number of cell lines analyzed. (b) Summary of somatic mutations affecting ZNF750 across different tumor types including mutation data reported by The Cancer Genome Atlas (TCGA, see URL) and Catalogue Of Somatic Mutations In Cancer (COSMIC, see URL). Number in the parentheses indicates the number of cases sequenced. Concerning C-Mel/CSCC, in TCGA pathological reports, several cutaneous melanoma (C-Mel) patients with ZNF750 mutations also had cutaneous squamous cell carcinoma (CSCC). UASCC, upper aerodigestive squamous cell carcinoma; HNSCC, head and neck squamous cell carcinoma; CRC, colon and rectum adenocarcinoma; LG Glioma, lower grade glioma; AML, acute myeloid leukemia; GBM, glioblastoma multiforme; AG, autonomic ganglia carcinoma. Squamous cell carcinomas were highlighted with Orange Square. (c) Upper panel, mRNA expression of indicated terminal differentiation-related genes quantified with q-PCR in EC109 and KYSE180 cells transfected with either siRNAs against ZNF750 (siZNF750) or control siRNA (Scramble), as well as (lower panel) infected with lentivirus encoding either of ZNF750 protein (Flag-ZNF750) or GFP (GFP-control). WB results showing ZNF750 protein level in indicated samples. β-Actin was used as a loading control. Value represent mean ± s.d. N = 5. (d) Short-term cell proliferation assay of ESCC cells treated with control medium (DMSO) or TPA (100nM) for 5 days. N = 3. **, P < 0.01; *, P < 0.05.
Supplementary Figure 6 FAT gene mutations and loss of heterozygosity.
(a) Summary of somatic mutations affecting FAT1, FAT2 and FAT3 across different tumor types from COSMIC. Number in the parentheses indicates the number of cases sequenced. (b) Inactivation of both alleles of FAT1 in two ESCC cases. Upper panel, SNP-array data showing FAT1 loss of heterozygosity in both tumors (ESCC-D10 and ESCC-D20, which were from Discovery Cohort). Blue line, total gene dosage; Green and red line, alleles-specific gene dosage. The number on the left indicates log2 tumor/reference ratio. Lower panel, Sanger sequencing signals confirmed the somatic mutations of FAT1 in both cases. Arrows indicate the beginning of frameshift mutation bases in tumor DNA and the corresponding bases in germline DNA.
Supplementary Figure 7 The growth-suppressive properties of FAT1 in ESCC cells.
KYSE150 cells stably expressing either FAT1 protein (Flag-FAT1) or GFP (GFP-control) were subjected to (a) short-term cell proliferation assay or (b) soft-agar colony formation assay. (c) WB results showing FAT1 protein level. β-Actin was used as a loading control. Value represent mean ± SD. N = 3. **, P < 0.01. *, P < 0.05.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–13 (PDF 15766 kb)
Supplementary Table 14
Chromosome regions for targeted capture with SureSelect cRNA baits (Frequency Cohort). (XLSX 899 kb)
Source data
Rights and permissions
About this article
Cite this article
Lin, DC., Hao, JJ., Nagata, Y. et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet 46, 467–473 (2014). https://doi.org/10.1038/ng.2935
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2935
This article is cited by
-
Understanding PI3K/Akt/mTOR signaling in squamous cell carcinoma: mutated PIK3CA as an example
Molecular Biomedicine (2024)
-
Histone 3 lysine 9 acetylation-specific reprogramming regulates esophageal squamous cell carcinoma progression and metastasis
Cancer Gene Therapy (2024)
-
circFNDC3B promotes esophageal squamous cell carcinoma progression by targeting MYO5A via miR-370-3p/miR-136-5p
BMC Cancer (2023)
-
Targeting IGF1R signaling enhances the sensitivity of cisplatin by inhibiting proline and arginine metabolism in oesophageal squamous cell carcinoma under hypoxia
Journal of Experimental & Clinical Cancer Research (2023)
-
FBXW7 loss of function promotes esophageal squamous cell carcinoma progression via elevating MAP4 and ERK phosphorylation
Journal of Experimental & Clinical Cancer Research (2023)