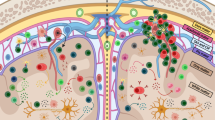
Abstract
Multiple sclerosis (MS) is a chronic disease of the CNS that is characterized by inflammation, demyelination and axonal injury. Although the etiology of MS is still unknown, many findings point toward a central role for the immune system in the pathogenesis of the disease. This hypothesis is strongly supported by the beneficial effects of immunomodulatory and immunosuppressive therapy on disease activity. Over the past few years, substantial progress has been made in deciphering the immune response in MS. Although animal models have advanced our knowledge of basic mechanisms of immune responses in the CNS, recent studies have also highlighted the differences between MS and its animal equivalent, experimental autoimmune encephalomyelitis. New immunotherapeutic agents have been developed and evaluated in clinical trials. Here, we review current knowledge of the immunopathogenesis of MS and corresponding animal models of disease, and discuss new immunointerventional treatment strategies based on changing pathogenetic concepts.
Key Points
-
The etiology of multiple sclerosis (MS) is unknown, but many findings indicate a central role for the immune system in the disease pathogenesis, and both genes and environmental factors influence the risk of developing disease
-
The most extensively studied animal model of MS is experimental autoimmune encephalomyelitis, a T-cell-mediated inflammatory disease of the CNS with variable degrees of demyelination and axonal damage
-
Traditionally, MS has been considered primarily to be a T-cell-mediated disease, but the importance of B lymphocytes in the pathogenesis of MS is beginning to be appreciated
-
There are important differences between the pathology of MS in humans and EAE in animals, particularly with regard to the involvement of B cells and CD8+ T cells
-
Targeting of the immune response is the most widely used treatment approach for MS; strategies range from nonselective immunosuppression to highly specific immune intervention
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Noseworthy JH et al. (2000) Multiple sclerosis. N Engl J Med 343: 938–952
Sadovnick AD et al. (1996) Evidence for genetic basis of multiple sclerosis. The Canadian Collaborative Study Group. Lancet 347: 1728–1730
Olerup O and Hillert J (1991) HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens 38: 1–15
Gale CR and Martyn CN (1995) Migrant studies in multiple sclerosis. Prog Neurobiol 47: 425–448
Kurtzke JF (2000) Epidemiology of multiple sclerosis: does this really point toward an etiology? Lectio Doctoralis. Neurol Sci 21: 383–403
Buljevac D et al. (2002) Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain 125: 952–960
Cserr HF and Knopf PM (1992) Cervical lymphatics, the blood–brain barrier and the immunoreactivity of the brain: a new view. Immunol Today 13: 507–512
Heppner FL et al. (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11: 146–152
Greter M et al. (2005) Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med 11: 328–334
Neumann H et al. (1995) Induction of MHC class I genes in neurons. Science 269: 549–552
Dandekar AA et al. (2001) Axonal damage is T cell mediated and occurs concomitantly with demyelination in mice infected with a neurotropic coronavirus. J Virol 75: 6115–6120
Kawakami N et al. (2005) Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion. J Exp Med 201: 1805–1814
Alter A et al. (2003) Determinants of human B cell migration across brain endothelial cells. J Immunol 170: 4497–4505
Uccelli A et al. (2005) Unveiling the enigma of the CNS as a B-cell fostering environment. Trends Immunol 26: 254–259
Zamvil SS and Steinman L (1990) The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol 8: 579–621
Linington C et al. (1988) Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 130: 443–454
Huseby ES et al. (2001) A pathogenic role for myelin-specific CD8+ T cells in a model for multiple sclerosis. J Exp Med 194: 669–676
Fujinami RS and Oldstone MB (1985) Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 230: 1043–1045
Wucherpfennig KW and Strominger JL (1995) Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80: 695–705
Hemmer B et al. (1997) Identification of high potency microbial and self ligands for a human autoreactive class II-restricted T cell clone. J Exp Med 185: 1651–1659
Lehmann PV et al. (1993) Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today 14: 203–208
Vanderlugt CL and Miller SD (2002) Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol 2: 85–95
Stohlman SA and Hinton DR (2001) Viral induced demyelination. Brain Pathol 11: 92–106
Richt JA et al. (1994) Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J Exp Med 179: 1467–1473
Ramakrishna C et al. (2002) Mechanisms of central nervous system viral persistence: the critical role of antibody and B cells. J Immunol 168: 1204–1211
Kerschensteiner M et al. (2003) Neurotrophic cross-talk between the nervous and immune systems: implications for neurological diseases. Ann Neurol 53: 292–304
Moalem G et al. (1999) Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med 5: 49–55
Jones TB et al. (2004) Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci 24: 3752–3761
Hohlfeld R and Wiendl H (2001) The ups and downs of multiple sclerosis therapeutics. Ann Neurol 49: 281–284
Gay FW et al. (1997) The application of multifactorial cluster analysis in the staging of plaques in early multiple sclerosis: identification and characterization of the primary demyelinating lesion. Brain 120: 1461–1483
Cannella B and Raine CS (1995) The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol 37: 424–435
Trebst C and Ransohoff RM (2001) Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch Neurol 58: 1975–1980
Trapp BD et al. (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338: 278–285
Kuhlmann T et al. (2002) Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain 125: 2202–2212
Lucchinetti C et al. (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47: 707–717
Lucchinetti CF et al. (2002) A role for humoral mechanisms in the pathogenesis of Devic's neuromyelitis optica. Brain 125: 1450–1461
Barnett MH and Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55: 458–468
Sospedra M and Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23: 683–747
Pette M et al. (1990) Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology 40: 1770–1776
Madsen LS et al. (1999) A humanized model for multiple sclerosis using HLA-DR2 and a human T-cell receptor. Nat Genet 23: 343–347
Hemmer B et al. (1996) Cytokine phenotype of human autoreactive T cell clones specific for the immunodominant myelin basic protein peptide (83–99). J Neurosci Res 45: 852–862
Oksenberg JR et al. (1990) Limited heterogeneity of rearranged T-cell receptor V alpha transcripts in brains of multiple sclerosis patients. Nature 345: 344–346
Babbe H et al. (2000) Clonal expansions of CD8+ T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med 192: 393–404
Jacobsen M et al. (2002) Oligoclonal expansion of memory CD8+ T cells in the cerebrospinal fluid from multiple sclerosis patients. Brain 125: 538–550
Skulina C et al. (2004) Multiple sclerosis: brain-infiltrating CD8+ T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA 101: 2428–2433
Cross AH et al. (2001) B cells and antibodies in CNS demyelinating disease. J Neuroimmunol 112: 1–14
Qin Y et al. (1998) Clonal expansion and somatic hypermutation of VH genes of B cells from cerebrospinal fluid in multiple sclerosis. J Clin Invest 102: 1045–1050
Baranzini SE et al. (1999) B cell repertoire diversity and clonal expansion in multiple sclerosis brain lesions. J Immunol 163: 5133–5144
Colombo M et al. (2000) Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol 164: 2782–2789
Colombo M et al. (2003) Maintenance of B lymphocyte-related clones in the cerebrospinal fluid of multiple sclerosis patients. Eur J Immunol 33: 3433–3438
Krumbholz M et al. (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201: 195–200
Cepok S et al. (2005) Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain 128: 1667–1676
Corcione A et al. (2004) Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci USA 101: 11064–11069
Cepok S et al. (2003) The immune response at onset and during recovery from Borrelia burgdorferi meningoradiculitis. Arch Neurol 60: 849–855
Lennon VA et al. (2005) IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 202: 473–477
Lennon VA et al. (2004) A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 364: 2106–2112
Bjartmar C and Trapp BD (2001) Axonal and neuronal degeneration in multiple sclerosis: mechanisms and functional consequences. Curr Opin Neurol 14: 271–278
Levin LI et al. (2005) Temporal relationship between elevation of epstein–barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA 293: 2496–2500
Alotaibi S et al. (2004) Epstein–Barr virus in pediatric multiple sclerosis. JAMA 291: 1875–1879
Reindl M et al. (1999) Antibodies against the myelin oligodendrocyte glycoprotein and the myelin basic protein in multiple sclerosis and other neurological diseases: a comparative study. Brain 122: 2047–2056
Cepok S et al. (2005) Identification of Epstein–Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest 115: 1352–1360
Soldan SS et al. (1997) Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med 3: 1394–1397
Hartung HP et al. (2002) Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet 360: 2018–2025
Yong VW (2002) Differential mechanisms of action of interferon-β and glatiramer acetate in MS. Neurology 59: 802–808
Neuhaus O et al. (2000) Multiple sclerosis: comparison of copolymer-1-reactive T cell lines from treated and untreated subjects reveals cytokine shift from T helper 1 to T helper 2 cells. Proc Natl Acad Sci USA 97: 7452–7457
Weber MS et al. (2004) Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain 127: 1370–1378
[No authors listed] (1993) Interferon β-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. The IFN-β Multiple Sclerosis Study Group. Neurology 43: 655–661
Jacobs LD et al. (1996) Intramuscular interferon β-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39: 285–294
[No authors listed] (1998) Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 352: 1498–1504
Johnson KP et al. (1995) Copolymer 1 reduces relapse rate and improves disability in relapsing–remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 45: 1268–1276
[No authors listed] (1998) Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon β-1b in secondary progressive MS. Lancet 352: 1491–1497
Panitch H et al. (2004) Interferon β-1b in secondary progressive MS: results from a 3-year controlled study. Neurology 63: 1788–1795
Youssef S et al. (2002) The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature 420: 78–84
Vollmer T et al. (2004) Oral simvastatin treatment in relapsing–remitting multiple sclerosis. Lancet 363: 1607–1608
Metz LM et al. (2004) Minocycline reduces gadolinium-enhancing magnetic resonance imaging lesions in multiple sclerosis. Ann Neurol 55: 756
Burt RK et al. (2005) Hematopoietic stem cell transplantation for multiple sclerosis. Arch Neurol 62: 860–864
van Oosten BW et al. (1996) A phase II trial of anti-CD4 antibodies in the treatment of multiple sclerosis. Mult Scler 1: 339–342
Cree BA et al. (2005) An open label study of the effects of rituximab in neuromyelitis optica. Neurology 64: 1270–1272
Stuve O et al. (2005) Clinical stabilization and effective B cell depletion in the cerebrospinal fluid and peripheral blood in a patient with fulminant relapsing remitting multiple sclerosis. Arch Neurol 62: 1620–1623
Coles AJ et al. (1999) Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol 46: 296–304
Bielekova B et al. (2004) Humanized anti-CD25 (daclizumab) inhibits disease activity in multiple sclerosis patients failing to respond to interferon β. Proc Natl Acad Sci USA 101: 8705–8708
[No authors listed] (1999) The Lenercept Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group TNF neutralization in MS: results of a randomized, placebo-controlled multicenter study. Neurology 53: 457–465
Kappos L et al. (2000) Induction of a non-encephalitogenic type 2 T helper-cell autoimmune response in multiple sclerosis after administration of an altered peptide ligand in a placebo-controlled, randomized phase II trial. The Altered Peptide Ligand in Relapsing MS Study Group. Nat Med 6: 1176–1182
Bielekova B et al. (2000) Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat Med 6: 1167–1175
Garren H et al. (2001) Combination of gene delivery and DNA vaccination to protect from and reverse Th1 autoimmune disease via deviation to the Th2 pathway. Immunity 15: 15–22
Lobell A et al. (2003) Suppressive DNA vaccination in myelin oligodendrocyte glycoprotein peptide-induced experimental autoimmune encephalomyelitis involves a T1-biased immune response. J Immunol 170: 1806–1813
Vandenbark AA et al. (1996) Treatment of multiple sclerosis with T-cell receptor peptides: results of a double-blind pilot trial. Nat Med 2: 1109–1115
Engelhardt B and Ransohoff RM (2005) The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 26: 485–495
Polman CH et al. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354: 899–910
Adelman B et al. (2005) Natalizumab and progressive multifocal leukoencephalopathy. N Engl J Med 353: 432–433
Cyster JG (2005) Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol 23: 127–159
Pluchino S et al. (2005) Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436: 266–271
Acknowledgements
B Hemmer, S Nessler and D Zhou were supported by grants from the Deutsche Forschungs-gemeinschaft, the Gemeinnützige Hertie-Stiftung and the German MS Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
S Nessler and D Zhou declared they have no competing interests.
Bernhard Hemmer has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Biogen Idec, Sanofi-Aventis, Schering, Serono, Teva Pharmaceuticals, Wyeth and Amgen. Bernd Kieseier has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer, Biogen Idec, Sanofi-Aventis, Schering, Serono and Teva Pharmaceuticals. Hans-Peter Hartung has received honoraria for lectures and consulting from Bayer, Biogen Idec, Sanofi-Aventis, Schering, Serono, Teva Pharmaceuticals, Wyeth and Amgen.
Rights and permissions
About this article
Cite this article
Hemmer, B., Nessler, S., Zhou, D. et al. Immunopathogenesis and immunotherapy of multiple sclerosis. Nat Rev Neurol 2, 201–211 (2006). https://doi.org/10.1038/ncpneuro0154
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpneuro0154
This article is cited by
-
Platelets and platelet-derived vesicles as an innovative cellular and subcellular platform for managing multiple sclerosis
Molecular Biology Reports (2023)
-
The alterations of cerebrospinal fluid TNF-alpha and TGF-beta2 levels in early relapsing–remitting multiple sclerosis
Immunologic Research (2022)
-
Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders
Genes & Immunity (2020)
-
Daphnetin Ameliorates Experimental Autoimmune Encephalomyelitis Through Regulating Heme Oxygenase-1
Neurochemical Research (2020)
-
The attentional cost of movement in multiple sclerosis
Journal of Neural Transmission (2019)