Abstract
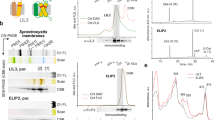
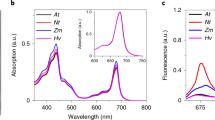
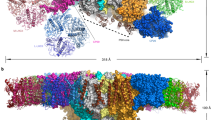
Plants collect light for photosynthesis using light-harvesting complexes (LHCs)—an array of chlorophyll proteins that are able to reversibly switch from harvesting to energy-dissipation mode to prevent damage of the photosynthetic apparatus. LHC antennae as well as other members of the LHC superfamily evolved from cyanobacterial ancestors called high light–inducible proteins (Hlips). Here, we characterized a purified Hlip family member HliD isolated from the cyanobacterium Synechocystis sp. PCC 6803. We found that the HliD binds chlorophyll-a (Chl-a) and β-carotene and exhibits an energy-dissipative conformation. Using femtosecond spectroscopy, we demonstrated that the energy dissipation is achieved via direct energy transfer from a Chl-a Qy state to the β-carotene S1 state. We did not detect any cation of β-carotene that would accompany Chl-a quenching. These results provide proof of principle that this quenching mechanism operates in the LHC superfamily and also shed light on the photoprotective role of Hlips and the evolution of LHC antennae.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Holt, N.E. et al. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307, 433–436 (2005).
Ruban, A.V. et al. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450, 575–578 (2007).
Bode, S. et al. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. USA 106, 12311–12316 (2009).
Avenson, T.J. et al. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 283, 3550–3558 (2008).
Fuciman, M. et al. Role of xanthophylls in light harvesting in green plants: a spectroscopic investigation of mutant LHCII and Lhcb pigment-protein complexes. J. Phys. Chem. B 116, 3834–3849 (2012).
Berera, R., van Stokkum, I.H.M., Kennis, J.T.M., van Grondelle, R. & Dekker, J.P. The light-harvesting function of carotenoids in the cyanobacterial stress-inducible IsiA complex. Chem. Phys. 373, 65–70 (2010).
Müller, M.G. et al. Singlet energy dissipation in the photosystem II light-harvesting complex does not involve energy transfer to carotenoids. ChemPhysChem 11, 1289–1296 (2010).
Dolganov, N.A.M., Bhaya, D. & Grossman, A.R. Cyanobacterial protein with similarity to the chlorophyll a/b binding proteins of higher plants: evolution and regulation. Proc. Natl. Acad. Sci. USA 92, 636–640 (1995).
Neilson, J.A.D. & Durnford, D.G. Structural and functional diversification of the light-harvesting complexes in photosynthetic eukaryotes. Photosynth. Res. 106, 57–71 (2010).
Engelken, J., Brinkmann, H. & Adamska, I. Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol. Biol. 10, 233 (2010).
Bhaya, D., Dufresne, A., Vaulot, D. & Grossman, A. Analysis of the hli gene family in marine and freshwater cyanobacteria. FEMS Microbiol. Lett. 215, 209–219 (2002).
He, Q., Dolganov, N., Bjorkman, O. & Grossman, A.R. The high light-inducible polypeptides in Synechocystis PCC6803. Expression and function in high light. J. Biol. Chem. 276, 306–314 (2001).
Chidgey, J.W. et al. A cyanobacterial chlorophyll synthase-HliD complex associates with the Ycf39 protein and the YidC/Alb3 insertase. Plant Cell 26, 1267–1279 (2014).
Vavilin, D., Yao, D. & Vermaas, W.F.J. Small cab-like proteins retard degradation of photosystem II-associated chlorophyll in Synechocystis sp PCC 6803—Kinetic analysis of pigment labeling with N-15 and C-13. J. Biol. Chem. 282, 37660–37668 (2007).
Yao, D. et al. Localization of the small CAB-like proteins in photosystem II. J. Biol. Chem. 282, 267–276 (2007).
Knoppová, J. et al. Discovery of a chlorophyll binding protein complex involved in the early steps of photosystem II assembly in Synechocystis. Plant Cell 26, 1200–1212 (2014).
Liu, Z. et al. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 428, 287–292 (2004).
Standfuss, J., Terwisscha van Scheltinga, A.C., Lamborghini, M. & Kühlbrandt, W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 Å resolution. EMBO J. 24, 919–928 (2005).
Polívka, T. & Sundström, V. Ultrafast dynamics of carotenoid excited states—from solution to natural and artificial systems. Chem. Rev. 104, 2021–2071 (2004).
Jeevarajan, J.A., Wei, C.C., Jeevarajan, A.S. & Kispert, L.D. Optical absorption spectra of dications of carotenoids. J. Phys. Chem. 100, 5637–5641 (1996).
Berera, R. et al. A mechanism of energy dissipation in cyanobacteria. Biophys. J. 96, 2261–2267 (2009).
Kosumi, D. et al. Ultrafast relaxation kinetics of the dark S-1 state in all-trans-β-carotene explored by one- and two-photon pump-probe spectroscopy. Chem. Phys. 373, 33–37 (2010).
Gradinaru, C.C. et al. An unusual pathway of excitation energy deactivation in carotenoids: singlet-to-triplet conversion on an ultrafast timescale in a photosynthetic antenna. Proc. Natl. Acad. Sci. USA 98, 2364–2369 (2001).
Tanaka, R. et al. LIL3, a light-harvesting-like protein, plays an essential role in chlorophyll and tocopherol biosynthesis. Proc. Natl. Acad. Sci. USA 107, 16721–16725 (2010).
Adamska, I., Roobol-Boza, M., Lindahl, M. & Andersson, B. Isolation of pigment-binding early light-inducible proteins from pea. Eur. J. Biochem. 260, 453–460 (1999).
Pan, X. et al. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 18, 309–315 (2011).
Krüger, T.P., Wientjes, E., Croce, R. & van Grondelle, R. Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc. Natl. Acad. Sci. USA 108, 13516–13521 (2011).
Koziol, A.G. et al. Tracing the evolution of the light-harvesting antennae in chlorophyll a/b-containing organisms. Plant Physiol. 143, 1802–1816 (2007).
Ruban, A.V., Young, A. & Horton, P. Modulation of chlorophyll fluorescence quenching in isolated light-harvesting complex of photosystem II. Biochim. Biophys. Acta 1186, 123–127 (1994).
Kaáa, R., Kotabová, E., Sobotka, R. & Prášil, O. Non-photochemical quenching in cryptophyte alga Rhodomonas salina is located in chlorophyll a/c antennae. PLoS ONE 7, e29700 (2012).
Bonente, G. et al. Analysis of LhcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 9, e1000577 (2011).
Xu, H., Vavilin, D., Funk, C. & Vermaas, W.F.J. Multiple deletions of small cab-like proteins in the cyanobacterium Synechocystis sp PCC 6803—Consequences for pigment biosynthesis and accumulation. J. Biol. Chem. 279, 27971–27979 (2004).
Wittig, I., Karas, M. & Schägger, H. High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell. Proteomics 6, 1215–1225 (2007).
Dobáková, M., Sobotka, R., Tichý, M. & Komenda, J. Psb28 protein is involved in the biogenesis of the photosystem II inner antenna CP47 (PsbB) in the cyanobacterium Synechocystis sp PCC 6803. Plant Physiol. 149, 1076–1086 (2009).
Boehm, M. et al. Investigating the early stages of Photosystem II assembly in Synechocystis sp PCC 6803. Isolation of CP47 and CP43 complexes. J. Biol. Chem. 286, 14812–14819 (2011).
Eijckelhoff, C. & Dekker, J.P. A routine method to determine the chlorophyll alpha, pheophytin-α and β-carotene contents of isolated Photosystem II reaction center complexes. Photosynth. Res. 52, 69–73 (1997).
Lakowicz, J.R. Principles of Fluorescence Spectroscopy 1st edn. (Kluwer Academic, 1999).
van Stokkum, I.H., Larsen, D.S. & van Grondelle, R. Global and target analysis of time-resolved spectra. Biochim. Biophys. Acta 1657, 82–104 (2004).
Acknowledgements
The authors thank M. Durchan and J. Tichý for their help with fluorescence measurements. J.K., T.P., V.Š. and R.S. were supported by the project P501/12/G055 from the Czech Science Foundation and by project Algatech. M.K.S. was supported by the project 14-13967S from the Czech Science Foundation and H.S. by the project CZ.1.07/2.3.00/30.0049.
Author information
Authors and Affiliations
Contributions
M.K.S. purified the f.Ycf39–HliD complex under the supervision of R.S.; J.K., R.K. and R.S. performed biochemical analyses. H.S. and V.Š. performed ultrafast spectroscopic experiments and analyzed data under the supervision of T.P.; R.S., T.P. and J.K. designed the study and wrote the paper. The whole study was supervised by R.S. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Table 1 and Supplementary Figures 1–8. (PDF 904 kb)
Rights and permissions
About this article
Cite this article
Staleva, H., Komenda, J., Shukla, M. et al. Mechanism of photoprotection in the cyanobacterial ancestor of plant antenna proteins. Nat Chem Biol 11, 287–291 (2015). https://doi.org/10.1038/nchembio.1755
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.1755
This article is cited by
-
Relationship between non-photochemical quenching efficiency and the energy transfer rate from phycobilisomes to photosystem II
Photosynthesis Research (2024)
-
Absolute quantification of cellular levels of photosynthesis-related proteins in Synechocystis sp. PCC 6803
Photosynthesis Research (2023)
-
Isomerization of carotenoids in photosynthesis and metabolic adaptation
Biophysical Reviews (2023)
-
High-light-inducible proteins HliA and HliB: pigment binding and protein–protein interactions
Photosynthesis Research (2022)
-
Psb34 protein modulates binding of high-light-inducible proteins to CP47-containing photosystem II assembly intermediates in the cyanobacterium Synechocystis sp. PCC 6803
Photosynthesis Research (2022)