Abstract
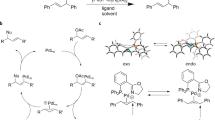
The power of natural selection through survival of the fittest is nature's ultimate tool for the improvement and advancement of species. To apply this concept in catalyst development is attractive and may lead to more rapid discoveries of new catalysts for the synthesis of relevant targets, such as pharmaceuticals. Recent advances in ligand synthesis using combinatorial methods have allowed the generation of a great diversity of catalysts. However, selection methods are few in number. We introduce a new selection method that focuses on the stability of catalytic intermediates measured by mass spectrometry. The stability of the intermediate relates inversely to the reactivity of the catalyst, which forms the basis of a catalyst-screening protocol in which less-abundant species represent the most-active catalysts, ‘the survival of the weakest’. We demonstrate this concept in the palladium-catalysed allylic alkylation reaction using diphosphine and IndolPhos ligands and support our results with high-level density functional theory calculations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Reetz, M. T. Combinatorial and evolution-based methods in the creation of enantioselective catalysts. Angew. Chem. Int. Ed. 40, 284–310 (2001).
Francis, M. B., Jamison, T. F. & Jacobsen, E. N. Combinatorial libraries of transition-metal complexes, catalysts and materials. Curr. Opin. Chem. Biol. 2, 422–428 (1998).
Reetz, M. T. Combinatorial transition-metal catalysis: mixing monodentate ligands to control enantio-, diastereo-, and regioselectivity. Angew. Chem. Int. Ed. 47, 2556–2588 (2008).
Goudriaan, P. E., van Leeuwen, P. W. N. M., Birkholz, M. N. & Reek, J. N. H. Libraries of bidentate phosphorus ligands; synthesis strategies and application in catalysis. Eur. J. Inorg. Chem. 2939–2958 (2008).
Wennemers, H. Combinatorial chemistry: a tool for the discovery of new catalysts. Comb. Chem. High Throughput Screen. 4, 273–285 (2001).
Krattiger, P., McCarthy, C., Pfaltz, A. & Wennemers, H. Catalyst–substrate coimmobilization: a strategy for catalysts discovery in split-and-mix libraries. Angew. Chem. Int. Ed. 42, 1722–1724 (2003).
Traverse, J. F. & Snapper, M. L. High-throughput methods for the development of new catalytic asymmetric reactions. Drug Discov. Today 7, 1002–1012 (2002).
Schultz, P. G. & Lerner, R. A. From molecular diversity to catalysis – lessons from the immune system. Science 269, 1835–1842 (1995).
Brisig, B., Sanders, J. K. M. & Otto, S. Selection and amplification of a catalyst from a dynamic combinatorial library. Angew. Chem. Int. Ed. 42, 1270–1273 (2003).
Polborn, K. & Severin, K. Molecular imprinting with an organometallic transition state analogue. Chem. Commun. 2481–2482 (1999).
Polborn, K. & Severin, K. Biomimetic catalysis with immobilised organometallic ruthenium complexes: substrate- and regioselective transfer hydrogenation of ketones. Chem. Eur. J. 6, 4604–4611 (2000).
Polborn, K. & Severin, K. Biomimetic catalysis with an immobilised chiral rhodium(iii) complex. Eur. J. Inorg. Chem. 1687–1692 (2000).
Markert, C. & Pfaltz, A. Screening of chiral catalysts and catalyst mixtures by mass spectrometric monitoring of catalytic intermediates. Angew. Chem. Int. Ed. 43, 2498–2500 (2004).
Markert, C., Neuburger, M., Kulicke, K., Meuwly, M. & Pfaltz, A. Palladium-catalyzed allylic substitution: reversible formation of allyl-bridged dinuclear palladium(i) complexes. Angew. Chem. Int. Ed. 46, 5892–5895 (2007).
Markert, C., Rosel, P. & Pfaltz, A. Combinatorial ligand development based on mass spectrometric screening and a double mass-labeling strategy. J. Am. Chem. Soc. 130, 3234–3235 (2008).
Teichert, A. & Pfaltz, A. Mass spectrometric screening of enantioselective Diels–Alder reactions. Angew. Chem. Int. Ed. 47, 3360–3362 (2008).
di Lena, F. & Matyjaszewski, K. Rapid screening of atom transfer radical polymerization catalysts by electrospray ionization mass spectrometry. Chem. Commun. 6306–6308 (2008).
di Lena, F. & Matyjaszewski, K. Investigation of metal ligand affinities of atom transfer radical polymerization catalysts with a quadrupole ion trap. Dalton Trans. 8884–8890 (2009).
Chen, P. Electrospray ionization tandem mass spectrometry in high-throughput screening of homogeneous catalysts. Angew. Chem. Int. Ed. 42, 2832–2847 (2003).
di Lena, F., Quintanilla, E. & Chen, P. Measuring rate constants for active species in the polymerization of ethylene by MAO-activated metallocene catalysts by electrospray ionization mass spectrometry. Chem. Commun. 5757–5759 (2005).
Quintanilla, E., di Lena, F. & Chen, P. Chain transfer to aluminium in MAO-activated metallocene-catalyzed polymerization reactions. Chem. Commun. 4309–4311 (2006).
Dietiker, R., di Lena, F. & Chen, P. Fourier transform ion mobility measurement of chain branching in mass-selected, chemically trapped oligomers from methylalumoxane-activated, metallocene-catalyzed polymerization of ethylene. J. Am. Chem. Soc. 129, 2796–2802 (2007).
Hinderling, C. & Chen, P. Rapid screening of olefin polymerization catalyst libraries by electrospray ionization tandem mass spectrometry. Angew. Chem. Int. Ed. 38, 2253–2256 (1999).
Hinderling, C. & Chen, P. Mass spectrometric assay of polymerization catalysts for combinational screening. Int. J. Mass Spectrom. 195-196, 377–383 (2000).
Di Marco, V. B. & Bombi, G. G. Electrospray mass spectrometry (ESI-MS) in the study of metal–ligand solution equilibria. Mass Spectrom. Rev. 25, 347–379 (2006).
Schalley, C. A. Supramolecular chemistry goes gas phase: the mass spectrometric examination of noncovalent interactions in host–guest chemistry and molecular recognition. Int. J. Mass Spectrom. 194, 11–39 (2000).
Wortmann, A. et al. Shrinking droplets in electrospray ionization and their influence on chemical equilibria. J. Am. Soc. Mass Spectrom. 18, 385–393 (2007).
Marcus, R. A. Theory of oxidation–reduction reactions involving electron transfer. 4. A statistical–mechanical basis for treating contributions from solvent, ligands, and inert salt. Discuss. Faraday Soc. 29, 21–31 (1960).
Bonchev, P. R. & Aleksiev, A. A. Use of Marcus's theory for selecting activators of homogeneous catalytic reactions. Theor. Exp. Chem. 9, 191–195 (1973).
Leussing, D. L. Successful application of Marcus theory to catalysis by labile metal ions. Transition Metal Chem. 23, 771–781 (1998).
Granberg, K. L. & Backvall, J. E. Isomerization of (pi-allyl)palladium complexes via nucleophilic displacement by palladium(0) – a common mechanism in palladium(0)-catalyzed allylic substitution. J. Am. Chem. Soc. 114, 6858–6863 (1992).
Evans, L. A. et al. Counterintuitive kinetics in Tsuji–Trost allylation: ion-pair partitioning and implications for asymmetric catalysis. J. Am. Chem. Soc. 130, 14471–14473 (2008).
Helmchen, G., Dahnz, A., Dubon, P., Schelwies, M. & Weihofen, R. Iridium-catalysed asymmetric allylic substitutions. Chem. Commun. 675–691 (2007).
Corbett, P. T. et al. Dynamic combinatorial chemistry. Chem. Rev. 106, 3652–3711 (2006).
van Haaren, R. J. et al. On the influence of the bite angle of bidentate phosphane ligands on the regioselectivity in allylic alkylation. Eur. J. Inorg. Chem. 1237–1241 (1999).
van Haaren, R. J. et al. An X-ray study of the effect of the bite angle of chelating ligands on the geometry of palladium(allyl) complexes: implications for the regioselectivity in the allylic alkylation. Inorg. Chem. 40, 3363–3372 (2001).
Atkins, P. & de Paula, J. Atkins's Physical Chemistry, 7th edn (Oxford Univ. Press, 2002).
te Velde, G. et al. Chemistry with ADF. J. Comput. Chem. 22, 931–967 (2001).
Bickelhaupt, F. M. & Baerends, E. J. in Reviews in Computational Chemistry Vol. 15 (eds Lipkowitz, K. B. & Boyd, D. B.) 1–86 (Wiley, 2000).
de Jong, G. T. & Bickelhaupt, F. M. Oxidative addition of the chloromethane C–Cl bond to Pd, an ab initio benchmark and DFT validation study. J. Chem. Theory Comput. 2, 322–335 (2006).
Wassenaar, J. et al. INDOLPhosphole and INDOLPhos palladium–allyl complexes in asymmetric allylic alkylations. Organometallics 28, 2724–2734 (2009).
Acknowledgements
This work was supported by the National Research School Combination – Catalysis, the European Union (RTN Revcat MRTN-CT-2006–035866) and the Netherlands Organization for Scientific Research (NWO-NCF and NWO-CW). The authors thank S. Ingemann and B. de Bruin for suggestions and discussions.
Author information
Authors and Affiliations
Contributions
J.W., E.J. and J.N.H.R. conceived and designed the experiments and analysed the data. W.-J.v.Z. and F.M.B. conceived and designed the DFT calculations and analysed the data. M.A.S. and A.L.S. determined the X-ray crystal structure of 2b. J.W. and E.J. performed the experiments. W.-J.v.Z. performed the DFT calculations. J.W., F.M.B. and J.N.H.R. wrote the paper, and all the authors edited and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 713 kb)
Supplementary information
Crystallographic data for compound 2b (CIF 64 kb)
Rights and permissions
About this article
Cite this article
Wassenaar, J., Jansen, E., van Zeist, WJ. et al. Catalyst selection based on intermediate stability measured by mass spectrometry. Nature Chem 2, 417–421 (2010). https://doi.org/10.1038/nchem.614
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.614
This article is cited by
-
Profiling the short-lived cationic species generated during catalytic dehydration of short-chain alcohols
Communications Chemistry (2018)
-
An autonomous organic reaction search engine for chemical reactivity
Nature Communications (2017)
-
Contemporary screening approaches to reaction discovery and development
Nature Chemistry (2014)
-
Label-assisted mass spectrometry for the acceleration of reaction discovery and optimization
Nature Chemistry (2013)
-
Three-component reaction discovery enabled by mass spectrometry of self-assembled monolayers
Nature Chemistry (2012)