Abstract
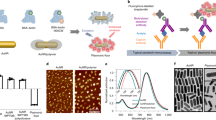
There is a direct correlation between protein levels and disease states in human serum, which makes it an attractive target for sensors and diagnostics. However, this is challenging because serum features more than 20,000 proteins, with an overall protein content greater than 1 mM. Here we report a sensor based on a hybrid synthetic–biomolecule that uses arrays of green fluorescent protein and nanoparticles to detect proteins at biorelevant concentrations in both buffer and human serum. Distinct and reproducible fluorescence-response patterns were obtained from five serum proteins (human serum albumin, immunoglobulin G, transferrin, fibrinogen and α-antitrypsin), both in buffer and when spiked into human serum. Using linear discriminant analysis we identified these proteins with an identification accuracy of 100% in buffer and 97% in human serum. The arrays were also able to discriminate between different concentrations of the same protein, as well as a mixture of different proteins in human serum.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Hardy, J. & Selkoe, D. J. Medicine – the amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002).
Pulido, R. & van Huijsduijnen, R. H. Protein tyrosine phosphatases: dual-specificity phosphatases in health and disease. FEBS J. 275, 848–866 (2008).
Adkins, J. N. et al. Toward a human blood serum proteome – analysis by multidimensional separation coupled with mass spectrometry. Mol. Cell. Proteomics 1, 947–955 (2002).
Pieper, R. et al. The human serum proteome: display of nearly 3700 chromatographically separated protein spots on two-dimensional electrophoresis gels and identification of 325 distinct proteins. Proteomics 3, 1345–1364 (2003).
Antman, E. M. et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N. Engl. J. Med. 335, 1342–1349 (1996).
Anderson, G. P. et al. Quantifying serum antiplague antibody with a fiber-optic biosensor. Clin. Diagn. Lab. Immunol. 5, 609–612 (1998).
Badgwell, D. & Bast, R. C. Early detection of ovarian cancer. Dis. Markers 23, 397–410 (2007).
Murphy, G. P., Elgamal, A. A. A., Su, S. L., Bostwick, D. G. & Holmes, E. H. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer 83, 2259–2269 (1998).
McPherson, R. A. & Pincus, M. R. Henry's Clinical Diagnosis and Management by Laboratory Methods Ch 19 (Saunders–Elsevier, 2007).
Li, J. N., Zhang, Z., Rosenzweig, J., Wang, Y. Y. & Chan, D. W. Proteomics and bioinformatics approaches for identification of serum biomarkers to detect breast cancer. Clin. Chem. 48, 1296–1304 (2002).
Baggerly, K. A., Morris, J. S. & Coombes, K. R. Reproducibility of SELDI–TOF protein patterns in serum: comparing datasets from different experiments. Bioinformatics 20, 777–U710 (2004).
Wright, A. T., Zhong, Z. & Anslyn, E. V. A functional assay for heparin in serum using a designed synthetic receptor. Angew. Chem. Int. Ed. 44, 5679–5682 (2005).
Tobey, S. L. & Anslyn, E. V. Determination of inorganic phosphate in serum and saliva using a synthetic receptor. Org. Lett. 5, 2029–2031 (2003).
Wright, A. T. & Anslyn, E. V. Differential receptor arrays and assays for solution-based molecular recognition. Chem. Soc. Rev. 35, 14–28 (2006).
Lavigne, J. J. & Anslyn, E. V. Sensing a paradigm shift in the field of molecular recognition: from selective to differential receptors. Angew. Chem. Int. Ed. 40, 3119–3130 (2001).
Wright, A. T., Edwards, N. Y., Anslyn, E. V. & McDevitt, J. T. The discriminatory power of differential receptor arrays is improved by prescreening – a demonstration in the analysis of tachykinins and similar peptides. Angew. Chem. Int. Ed. 46, 8212–8215 (2007).
Zhou, H. C., Baldini, L., Hong, J., Wilson, A. J. & Hamilton, A. D. Pattern recognition of proteins based on an array of functionalized porphyrins. J. Am. Chem. Soc. 128, 2421–2425 (2006).
Miranda, O. R. et al. Array-based sensing of proteins using conjugated polymers. J. Am. Chem. Soc. 129, 9856–9857 (2007).
Stephenson, C. J. & Shimizu, K. D. Colorimetric and fluorometric molecularly imprinted polymer sensors and binding assays. Polym. Int. 56, 482–488 (2007).
You, C. C. et al. Detection and identification of proteins using nanoparticle–fluorescent polymer ‘chemical nose’ sensors. Nature Nanotechnol. 2, 318–323 (2007).
Phillips, R. L., Miranda, O. R., You, C. C., Rotello, V. M. & Bunz, U. H. F. Rapid and efficient identification of bacteria using gold-nanoparticle–poly(para-phenyleneethynylene) constructs. Angew. Chem. Int. Ed. 47, 2590–2594 (2008).
De, M., You, C. C., Srivastava, S. & Rotello, V. M. Biomimetic interactions of proteins with functionalized nanoparticles: a thermodynamic study. J. Am. Chem. Soc. 129, 10747–10753 (2007).
Hong, R. et al. Control of protein structure and function through surface recognition by tailored nanoparticle scaffolds. J. Am. Chem. Soc. 126, 739–743 (2004).
Tsien, R. Y. The green fluorescent protein. Annu. Rev. Biochem. 67, 509–544 (1998).
Chalfie, M. & Kain, S. R. Green Fluorescent Protein: Properties, Applications, and Protocols p. 69 (Wiley-Interscience, 2006).
Anderson, N. L. & Anderson, N. G. The human plasma proteome – history, character, and diagnostic prospects. Mol. Cell. Proteomics 1, 845–867 (2002).
You, C. C., De, M., Han, G. & Rotello, V. M. Tunable inhibition and denaturation of alpha-chymotrypsin with amino acid-functionalized gold nanoparticles. J. Am. Chem. Soc. 127, 12873–12881 (2005).
Jurs, P. C., Bakken, G. A. & McClelland, H. E. Computational methods for the analysis of chemical sensor array data from volatile analytes. Chem. Rev. 100, 2649–2678 (2000).
De, M., Rana, S. & Rotello, V. M. Nickel-ion-mediated control of the stoichiometry of his-tagged protein/nanoparticle interactions. Macromol. Biosci. 9, 174–178 (2009).
SYSTAT 11.0 (Systat Software, Richmond, CA 94804, USA, 2004).
Acknowledgements
This work was supported by the National Science Foundation Center for Hierarchical Manufacturing at the University of Massachusetts and the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
M.D., S.R. and V.R. conceived and designed the experiments. M.D., S.R., H.A., O.M. and R.A. performed the experiments. M.D., S.R., O.M. and V.R. analysed the data. M.D., S.R., U.B. and V.R. co-wrote the paper.
Corresponding author
Supplementary information
Supplementary information
Supplementary information (PDF 5179 kb)
Rights and permissions
About this article
Cite this article
De, M., Rana, S., Akpinar, H. et al. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nature Chem 1, 461–465 (2009). https://doi.org/10.1038/nchem.334
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.334
This article is cited by
-
Isoelectric trapping and discrimination of histones from plasma in a microfluidic device using dehydrated isoelectric gate
Microchimica Acta (2024)
-
Chemical tongues: biomimetic recognition using arrays of synthetic polymers
Polymer Journal (2022)
-
Discrimination of deletion to point cytokine mutants based on an array of cross-reactive receptors mimicking protein recognition by heparan sulfate
Analytical and Bioanalytical Chemistry (2022)
-
Amino Acid Detection with Bare Eyes Based on Two Different Concentrations of Iodides as Sensor Receptors
Food Analytical Methods (2021)
-
A bioorthogonal system reveals antitumour immune function of pyroptosis
Nature (2020)