Abstract
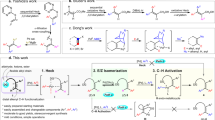
The ubiquitous nature of C–H bonds in organic molecules makes them attractive as a target for rapid complexity generation, but brings with it the problem of achieving selective reactions. In developing new methodologies for C–H functionalization, alkenes are an attractive starting material because of their abundance and low cost. Here we describe the conversion of 1-alkenes into 1,4-diols. The method involves the installation of a new Si,N-type chelating auxiliary group on the alkene followed by iridium-catalysed C–H silylation of an unactivated δ-C(sp3)–H bond to produce a silolane intermediate. Oxidation of the C–Si bonds affords a 1,4-diol. The method is demonstrated to have broad scope and good functional group compatibility by application to the selective 1,4-oxygenation of several natural products and derivatives.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yu, J-Q., Shi, Z. C–H Activation (Topics in Current Chemistry 292, Springer, 2010).
Engle, K. M., Mei, T-S., Wasa, M. & Yu, J-Q. Weak coordination as a powerful means for developing broadly useful C–H functionalization reactions. Acc. Chem. Res. 45, 788–802 (2011).
Wencel-Delord, J., Droge, T., Liu, F. & Glorius, F. Towards mild metal-catalyzed C–H bond activation. Chem. Soc. Rev. 40, 4740–4761 (2011).
Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C–H bond functionalizations: mechanism and scope. Chem. Rev. 111, 1315–1345 (2011).
Lyons, T. W. & Sanford, M. S. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 110, 1147–1169 (2010).
Labinger, J. A. & Bercaw, J. E. Understanding and exploiting C–H bond activation. Nature 417, 507–514 (2002).
Li, H., Li, B-J. & Shi, Z-J. Challenge and progress: palladium-catalyzed sp3 C–H activation. Catal. Sci. Technol. 1, 191–206 (2011).
Jazzar, R., Hitce, J., Renaudat, A., Sofack-Kreutzer, J. & Baudoin, O. Functionalization of organic molecules by transition-metal-catalyzed C(sp3)–H activation. Chem. Eur. J. 16, 2654–2672 (2010).
He, G., Zhao, Y., Zhang, S., Lu, C. & Chen, G. Highly efficient syntheses of azetidines, pyrrolidines, and indolines via palladium catalyzed intramolecular amination of C(sp3)–H and C(sp2)–H bonds at gamma and delta positions. J. Am. Chem. Soc. 134, 3–6 (2012).
Nadres, E. T. & Daugulis, O. Heterocycle synthesis via direct C–H/N–H coupling. J. Am. Chem. Soc. 134, 7–10 (2012).
White, M. C. Adding aliphatic C–H bond oxidations to synthesis. Science 335, 807–809 (2012).
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 50, 3362–3374 (2011).
Ortiz de Montellano, P. R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 110, 932–948 (2009).
McNeill, E. & Du Bois, J. Catalytic C–H oxidation by a triazamacrocyclic ruthenium complex. Chem. Sci. 3, 1810–1813 (2012).
Bigi, M. A., Reed, S. A. & White, M. C. Diverting non-haem iron catalysed aliphatic C–H hydroxylations towards desaturations. Nature Chem. 3, 216–222 (2011).
Prat, I. et al. Observation of Fe(V)=O using variable-temperature mass spectrometry and its enzyme-like C–H and C=C oxidation reactions. Nature Chem. 3, 788–793 (2011).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Kamata, K., Yonehara, K., Nakagawa, Y., Uehara, K. & Mizuno, N. Efficient stereo- and regioselective hydroxylation of alkanes catalysed by a bulky polyoxometalate. Nature Chem. 2, 478–483 (2010).
Chen, M. S. & White, M. C. A predictably selective aliphatic C–H oxidation reaction for complex molecule synthesis. Science 318, 783–787 (2007).
Das, S., Incarvito, C. D., Crabtree, R. H. & Brudvig, G. W. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science 312, 1941–1943 (2006).
Giri, R. et al. Pd-catalyzed stereoselective oxidation of methyl groups by inexpensive oxidants under mild conditions: a dual role for carboxylic anhydrides in catalytic C–H bond oxidation. Angew. Chem. Int. Ed. 44, 7420–7424 (2005).
Desai, L. V., Hull, K. L. & Sanford, M. S. Palladium-catalyzed oxygenation of unactivated sp3 C–H bonds. J. Am. Chem. Soc. 126, 9542–9543 (2004).
Simmons, E. M. & Hartwig, J. F. Catalytic functionalization of unactivated primary C–H bonds directed by an alcohol. Nature 483, 70–73 (2012).
Tsukada, N. & Hartwig, J. F. Intermolecular and intramolecular, platinum-catalyzed, acceptorless dehydrogenative coupling of hydrosilanes with aryl and aliphatic methyl C–H bonds. J. Am. Chem. Soc. 127, 5022–5023 (2005).
Kuninobu, Y., Nakahara, T., Takeshima, H. & Takai, K. Rhodium-catalyzed intramolecular silylation of unactivated C(sp3)–H bonds. Org. Lett. 15, 426–428 (2013).
Jones, G. R. & Landais, Y. The oxidation of the carbon–silicon bond. Tetrahedron 52, 7599–7662 (1996).
Kuznetsov, A. & Gevorgyan, V. General and practical one-pot synthesis of dihydrobenzosiloles from styrenes. Org. Lett. 14, 914–917 (2012).
Zaitsev, V. G., Shabashov, D. & Daugulis, O. Highly regioselective arylation of sp3 C–H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 127, 13154–13155 (2005).
Watanabe, H., Aoki, M., Sakurai, N., Watanabe, K-I. & Nagai, Y. Selective synthesis of mono-alkyldichlorosilanes via the reaction of olefins with dichlorosilane catalyzed by group VIII metal phosphine complexes. J. Organomet. Chem. 160, C1–C7 (1978).
Smitrovich, J. H. & Woerpel, K. A. Oxidation of sterically hindered alkoxysilanes and phenylsilanes under basic conditions. J. Org. Chem. 61, 6044–6046 (1996).
Wencel-Delord, J. & Glorius, F. C–H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nature Chem. 5, 369–375 (2013).
Acknowledgements
The support of the National Institute of Health (GM-64444) and the National Science Foundation (CHE-1112055) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
N.G., F.S.M. and A.V.G. contributed equally to this work. N.G., F.S.M. and A.V.G. designed and performed the experiments and wrote the manuscript. C.H. performed the experiments at an early stage of the project. All authors participated in the discussion of the results. V.G. conceived and guided the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ghavtadze, N., Melkonyan, F., Gulevich, A. et al. Conversion of 1-alkenes into 1,4-diols through an auxiliary-mediated formal homoallylic C–H oxidation. Nature Chem 6, 122–125 (2014). https://doi.org/10.1038/nchem.1841
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1841
This article is cited by
-
Nickel-catalysed retro-hydroamidocarbonylation of aliphatic amides to olefins
Nature Communications (2017)
-
Mesoporous MnCeOx solid solutions for low temperature and selective oxidation of hydrocarbons
Nature Communications (2015)
-
Direct silylation reactions of inert C-H bonds via transition metal catalysis
Science China Chemistry (2015)
-
A surrogate for selectivity
Nature Chemistry (2014)