Abstract
A crucial yet ill-defined step during the development of tubular networks, such as the vasculature, is the formation of connections (anastomoses) between pre-existing lumenized tubes. By studying tracheal tube anastomosis in Drosophila melanogaster, we uncovered a key role of secretory lysosome-related organelle (LRO) trafficking in lumen fusion. We identified the conserved calcium-binding protein Unc-13-4/Staccato (Stac) and the GTPase Rab39 as critical regulators of this process. Stac and Rab39 accumulate on dynamic vesicles, which form exclusively in fusion tip cells, move in a dynein-dependent manner, and contain late-endosomal, lysosomal, and SNARE components characteristic of LROs. The GTPase Arl3 is necessary and sufficient for Stac LRO formation and promotes Stac-dependent intracellular fusion of juxtaposed apical plasma membranes, thereby forming a transcellular lumen. Concomitantly, calcium is released locally from ER exit sites and apical membrane-associated calcium increases. We propose that calcium-dependent focused activation of LRO exocytosis restricts lumen fusion to appropriate domains within tip cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Blum, Y. et al. Complex cell rearrangements during intersegmental vessel sprouting and vessel fusion in the zebrafish embryo. Dev. Biol. 316, 312–322 (2008).
Herwig, L. et al. Distinct cellular mechanisms of blood vessel fusion in the zebrafish embryo. Curr. Biol. 21, 1942–1948 (2011).
Lenard, A. et al. In vivo analysis reveals a highly stereotypic morphogenetic pathway of vascular anastomosis. Dev. Cell 25, 492–506 (2013).
Gervais, L., Lebreton, G. & Casanova, J. The making of a fusion branch in the Drosophila trachea. Dev. Biol. 362, 187–193 (2012).
Caviglia, S. & Luschnig, S. Tube fusion: making connections in branched tubular networks. Semin. Cell Dev. Biol. 31C, 82–90 (2014).
Lubarsky, B. & Krasnow, M. A. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28 (2003).
Samakovlis, C. et al. Genetic control of epithelial tube fusion during Drosophila tracheal development. Development 122, 3531–3536 (1996).
Siekmann, A. F., Affolter, M. & Belting, H. G. The tip cell concept 10 years after: new players tune in for a common theme. Exp. Cell Res. 319, 1255–1263 (2013).
Lee, S. & Kolodziej, P. A. The plakin Short Stop and the RhoA GTPase are required for E-cadherin-dependent apical surface remodeling during tracheal tube fusion. Development 129, 1509–1520 (2002).
Lee, M., Lee, S., Zadeh, A. D. & Kolodziej, P. A. Distinct sites in E-cadherin regulate different steps in Drosophila tracheal tube fusion. Development 130, 5989–5999 (2003).
Tanaka, H. et al. Formin3 is required for assembly of the F-actin structure that mediates tracheal fusion in Drosophila. Dev. Biol. 274, 413–425 (2004).
Uv, A., Cantera, R. & Samakovlis, C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 13, 301–309 (2003).
Fededa, J. P. & Gerlich, D. W. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 14, 440–447 (2012).
Andrews, N. W., Almeida, P. E. & Corrotte, M. Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 24, 734–742 (2014).
Kakihara, K., Shinmyozu, K., Kato, K., Wada, H. & Hayashi, S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 small GTPase in Drosophila. Mech. Dev. 125, 325–336 (2008).
Marks, M. S., Heijnen, H. F. & Raposo, G. C. P. Lysosome-related organelles: unusual compartments become mainstream. Curr. Opin. Cell Biol. 25, 495–505 (2013).
Jiang, L., Rogers, S. L. & Crews, S. T. The Drosophila Dead end Arf-like3 GTPase controls vesicle trafficking during tracheal fusion cell morphogenesis. Dev. Biol. 311, 487–499 (2007).
Feldmann, J. et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3). Cell 115, 461–473 (2003).
Neeft, M. et al. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol. Biol. Cell 16, 731–741 (2005).
Tanaka-Matakatsu, M., Uemura, T., Oda, H., Takeichi, M. & Hayashi, S. Cadherin-mediated cell adhesion and cell motility in Drosophila trachea regulated by the transcription factor Escargot. Development 122, 3697–3705 (1996).
Uemura, T. et al. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 10, 659–671 (1996).
Zhang, L. & Ward, R. E. IV uninflatable encodes a novel ectodermal apical surface protein required for tracheal inflation in Drosophila. Dev. Biol. 336, 201–212 (2009).
Jazwinska, A. & Affolter, M. A family of genes encoding zona pellucida (ZP) domain proteins is expressed in various epithelial tissues during Drosophila embryogenesis. Gene Expr. Patterns 4, 413–421 (2004).
Koch, H., Hofmann, K. & Brose, N. C. P. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem. J. 349, 247–253 (2000).
Stenmark, H. Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol. 10, 513–525 (2009).
Dunst, S. et al. Endogenously tagged rab proteins: a resource to study membrane trafficking in Drosophila. Dev. Cell 33, 351–365 (2015).
Dong, B., Kakihara, K., Otani, T., Wada, H. & Hayashi, S. Rab9 and retromer regulate retrograde trafficking of luminal protein required for epithelial tube length control. Nat. Commun. 4, 1358 (2013).
Zhang, J. et al. Thirty-one flavors of Drosophila rab proteins. Genetics 176, 1307–1322 (2007).
Andrei, C. et al. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 10, 1463–1475 (1999).
Becker, C. E., Creagh, E. M. & O’Neill, L. A. C. P. Rab39a binds caspase-1 and is required for caspase-1-dependent interleukin-1β secretion. J. Biol. Chem. 284, 34531–34537 (2009).
Seto, S., Tsujimura, K. & Koide, Y. Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 12, 407–420 (2011).
Pulipparacharuvil, S. et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J. Cell Sci. 118, 3663–3673 (2005).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Stinchcombe, J. C., Majorovits, E., Bossi, G., Fuller, S. & Griffiths, G. M. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 443, 462–465 (2006).
Gervais, L. & Casanova, J. In vivo coupling of cell elongation and lumen formation in a single cell. Curr. Biol. 20, 359–366 (2010).
Boswell, K. L. et al. Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J. Cell Biol. 197, 301–312 (2012).
Ma, C., Li, W., Xu, Y. & Rizo, J. C. Munc13 mediates the transition from the closed syntaxin–Munc18 complex to the SNARE complex. Nat. Struct. Mol. Biol. 18, 542–549 (2011).
Laulagnier, K. et al. Role of AP1 and Gadkin in the traffic of secretory endo-lysosomes. Mol. Biol. Cell 22, 2068–2082 (2011).
Förster, D., Armbruster, K. & Luschnig, S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr. Biol. 20, 62–68 (2010).
Menager, M. M. et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat. Immunol. 8, 257–267 (2007).
Elstak, E. D. et al. Munc13-4∗rab27 complex tethers secretory lysosomes at the plasma membrane. Commun. Integr. Biol. 5, 64–67 (2012).
van Weering, J. R. & Cullen, P. J. Membrane-associated cargo recycling by tubule-based endosomal sorting. Semin. Cell Dev. Biol. 31C, 40–47 (2014).
Strilic, B. et al. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr. Biol. 20, 2003–2009 (2010).
Ismail, S. A. et al. Arl2-GTP and Arl3-GTP regulate a GDI-like transport system for farnesylated cargo. Nat. Chem. Biol. 7, 942–949 (2011).
Zhou, C., Cunningham, L., Marcus, A. I., Li, Y. & Kahn, R. A. C. P. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol. Biol. Cell 17, 2476–2487 (2006).
Wätzlich, D. et al. The interplay between RPGR, PDEd and Arl2/3 regulate the ciliary targeting of farnesylated cargo. EMBO Rep. 14, 465–472 (2013).
Parekh, A. B. Decoding cytosolic Ca2 + oscillations. Trends Biochem. Sci. 36, 78–87 (2011).
Kanamori, T. et al. Compartmentalized calcium transients trigger dendrite pruning in Drosophila sensory neurons. Science 340, 1475–1478 (2013).
Witze, E. S. et al. Wnt5a directs polarized calcium gradients by recruiting cortical endoplasmic reticulum to the cell trailing edge. Dev. Cell 26, 645–657 (2013).
Lee, T. & Luo, L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999).
Duncan, J. E. & Warrior, R. The cytoplasmic dynein and kinesin motors have interdependent roles in patterning the Drosophila oocyte. Curr. Biol. 12, 1982–1991 (2002).
Luschnig, S., Bätz, T., Armbruster, K. & Krasnow, M. serpentine and vermiform encode matrix proteins with chitin binding and deacetylation domains that limit tracheal tube length in Drosophila. Curr. Biol. 16, 186–194 (2006).
Bischof, J., Maeda, R. K., Hediger, M., Karch, F. & Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl Acad. Sci. USA 104, 3312–3317 (2007).
Riedl, J. et al. Lifeact: a versatile marker to visualize F-actin. Nat. Methods 5, 605–607 (2008).
Melom, J. E. & Littleton, J. T. C. P. Mutation of a NCKX eliminates glial microdomain calcium oscillations and enhances seizure susceptibility. J. Neurosci. 33, 1169–1178 (2013).
Allen, M. J. et al. Targeted expression of truncated glued disrupts giant fiber synapse formation in Drosophila. J. Neurosci. 19, 9374–9384 (1999).
Kohl, J. et al. Ultrafast tissue staining with chemical tags. Proc. Natl Acad. Sci. USA 111, E3805–E3814 (2014).
Kay, J. N. et al. Transient requirement for ganglion cells during assembly of retinal synaptic layers. Development 131, 1331–1342 (2004).
Jiang, L., Pearson, J. C. & Crews, S. T. Diverse modes of Drosophila tracheal fusion cell transcriptional regulation. Mech. Dev. 127, 265–280 (2010).
Sharma, Y., Cheung, U., Larsen, E. W. & Eberl, D. F. C. P. PPTGAL, a convenient Gal4 P-element vector for testing expression of enhancer fragments in Drosophila. Genesis 34, 115–118 (2002).
Ejsmont, R. K., Sarov, M., Winkler, S., Lipinski, K. A. & Tomancak, P. A toolkit for high-throughput, cross-species gene engineering in Drosophila. Nat. Methods 6, 435–437 (2009).
Sarov, M. et al. A genome-wide resource for the analysis of protein localisation in Drosophila. eLife 5, http://dx.doi.org/10.7554/eLife.12068 (2016).
Venken, K. J. et al. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat. Methods 8, 737–743 (2011).
Patel, N. H. Imaging neuronal subsets and other cell types in whole-mount Drosophila embryos and larvae using antibody probes. Methods Cell Biol. 44, 445–487 (1994).
Jiang, L. & Crews, S. T. The Drosophila dysfusion basic helix-loop-helix (bHLH)-PAS gene controls tracheal fusion and levels of the trachealess bHLH-PAS protein. Mol. Cell Biol. 23, 5625–5637 (2003).
Caviglia, S. & Luschnig, S. The ETS domain transcriptional repressor Anterior open inhibits MAP kinase and Wingless signaling to couple tracheal cell fate with branch identity. Development 140, 1240–1249 (2013).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Costes, S. V. et al. Automatic and quantitative measurement of protein–protein colocalization in live cells. Biophys. J. 86, 3993–4003 (2004).
Acknowledgements
We are indebted to D. Förster for isolating the stac3B20 mutant. We thank M. Trost for generating dys–Gal4, J. Shah for generating Stac–sGFP, J. Glashauser for generating UAS–palm-mKate2, M. Robinson for advice on statistics, and W. Backer for technical help. J. Bressan, M. Crameri, G. Gut, Z. Kabakci, T. Kohlbrenner, S. Re, J. Shah and M. Whitehead contributed experimental results. We thank M. Affolter, H. Chanut-Delalande (Université Paul Sabatier, Toulouse, France), D. Brunner, C. Lehner (University of Zurich, Switzerland), B. Dong, S. Hayashi (Riken Center for Developmental Biology, Kobe, Japan), G. Jefferis (University of Cambridge, UK), L. Jiang (Oakland University, USA), H. Krämer (UT Southwestern, USA), T. Littleton (MIT, USA), P. Tomancak (Max-Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany), the Bloomington and Kyoto Drosophila stock centres, and the Developmental Studies Hybridoma Bank for providing fly stocks and reagents. We thank B. Dong and S. Hayashi for communication of unpublished work. We are indebted to C. Lehner for continuous support and discussions. S.C. was supported by a Forschungskredit fellowship of the University of Zurich. Work in S.L.’s laboratory was supported by the Swiss National Science Foundation (SNF 31003A_141093_1), the University of Zurich, the Kanton Zurich, the ‘Cells-in-Motion’ Cluster of Excellence (EXC 1003-CiM), and the University of Münster.
Author information
Authors and Affiliations
Contributions
S.C. and S.L. designed and interpreted the experiments and wrote the manuscript. S.C. conducted the experiments and analysed the data. E.J.F. contributed to the analysis of Stac expression. M.B. and S.E. provided YFP–Rab stocks, help with the YFP–Rab localization screen, and co-localization experiments in fixed embryos. All authors contributed comments and critically read the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 4 staccato mutations affect a late step of the tube fusion process.
(a) Immunostaining of stage 15 embryos labeled with anti-Dys (green in merge) and a probe to detect chitin (magenta). Dys protein is present in FC nuclei in wild-type and stac3B20 mutant embryos. Note the interrupted tracheal lumen in the stac3B20 embryo. Images are representative of six embryos. (b) During contact formation between FCs, E-cadherin (E-cad, cyan), F-actin (magenta) and Short Stop (Shot, yellow) accumulate at the FC-FC interface. All three markers are present and correctly localised in wild-type and stac3B20 mutant embryos. Images are representative of >10 embryos from two experiments. (c) A cytoskeletal track (visualised by α-Tubulin staining, green) extends in the central region of each FC along the luminal axis in wild-type and in stac3B20 mutant embryos. Images are representative of >10 embryos. (d,e) The FC-specific zona pellucida protein CG13196 (green) is localised to FC apical membranes during late fusion. After fusion, in wild-type DT (d) and DB (e) FCs, CG13196 is detectable as rings surrounding the newly connected lumen. In stac3B20 embryos CG13196 is present on the primary apical membranes and surrounding isolated pockets of luminal material at FC-FC interfaces (white arrowheads), indicating that apical trafficking of CG13196 (and of Uif, see Fig. 1) is not perturbed. Images are representative of 10 embryos. (f) Arf-like 3 (Arl3, green) is specifically expressed in FCs and localises to puncta in wild-type and in stac3B20 mutant FCs. Images are representative of nine embryos from two experiments. In panels b,c and e the FC-FC interface is indicated by a white arrowhead. In panels c and f deconvolution was applied. Scale bars, 10 μm.
Supplementary Figure 5 The stac3B20 mutation gives rise to aberrantly spliced mRNAs and an unstable protein product.
(a) Reverse transcriptase PCRs on mRNA of wild-type and stac3B20 embryos using two different primer pairs. In the first two lanes (RT-PCR1) the reverse primer anneals to the mutated exon-intron junction in stac3B20. Only in this sample a PCR product was detectable, indicating that the last intron is retained in stac3B20 mutant mRNAs. A control primer pair (RT-PCR2) yields a product of an upstream part of the mRNA in stac3B20 and wild-type (WT) mRNA. A schematic representation of the primer binding sites on wild-type and stac3B20 mutant mRNAs is shown below the image. A DNA size marker is in the lane to the left (size of bands in bp is indicated). The PCR was repeated two times. (b) Live imaging of stac3B20 embryos expressing specifically in FCs the luminal protein Verm-RFP and cytoplasmic GFP (control), EGFP-StacA, or the mutant version EGFP-StacA3B20. Whereas wild-type EGFP-StacA rescues the luminal interruptions in control embryos, expression of EGFP-StacA3B20 fails to rescue lumen fusion defects (compare lower panel to upper control panels). (c) EGFP-StacA or EGFP-StacA3B20 were expressed throughout the tracheal system using btl-Gal4. DBs of living embryos are shown. Whereas wild-type EGFP-StacA (left panel) is readily detectable in all tracheal cells, EGFP-StacA3B20 (middle panel) is not detectable using the same acquisition settings. At increased laser power, EGFP-StacA3B20 signals become detectable in tracheal cells (right panel). FCs are indicated by red asterisks. Images are representative of nine embryos. (d) Close-up view of tracheal dorsal trunk in a genetic mosaic embryo carrying stac3B20 homozygous mutant tracheal cells marked by cytoplasmic GFP (cyan). Luminal chitin is labeled in magenta. Luminal interruptions were observed when fusion points contained at least one stac3B20 mutant FC, also in cases (n = 2) where the stac3B20 mutant FC (white arrowhead) was surrounded exclusively by heterozygous (stac3B20/+) or wild-type (+/+) cells, indicating that stac function is required in FCs for successful lumen fusion. Images are representative of eight embryos from two experiments. Scale bars, b 50 μm; c,d 10 μm.
Supplementary Figure 6 Embryonic expression pattern and subcellular localisation of Stac protein expressed at endogenous levels.
(a) Live imaging of stage 16–17 embryos expressing EGFP-tagged Stac at endogenous levels (Stac-sGFP). Stac-sGFP expression is detectable in epidermis, tracheal system (upper middle panel, close-up of the region indicated by the dashed rectangle shown in the upper right panel), Malpighian tubules, hemocytes, boundary cells of the hindgut, muscles and dorsal vessel. Hemocytes contained large Stac-sGFP-positive puncta, reflecting either phagocytosed GFP-positive cells from other tissues, or expression of Stac in hemocytes. Stac-sGFP marks vesicle-like structures in all tissues that show detectable Stac-sGFP expression. Images are representative of >20 embryos. (b) Stac-sGFP (green) labels membrane compartments localised distal to the primary apical domain (white arrowheads) in DB FCs. Tracheal cell membranes are marked by palmitoylated mKate2 (palm-mKate2, magenta). Images are representative of >10 embryos. Scale bars, a 50 μm; a (lower panels), b 10 μm. In panel b deconvolution was applied.
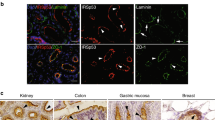
Supplementary Figure 7 Distribution of endogenously tagged Rab proteins in tracheal cells.
Rab proteins fall into three classes based on their distribution in FCs: Class I YRabs were enriched on intracellular membranes not overlapping with Stac vesicles and included YRab1, YRab2, YRab4, YRab5, YRab10, YRab18 and YRab19. Class II YRabs were enriched at apical membranes of tracheal cells and included YRab11 and YRab23. Class III YRabs were enriched in the central track region in FCs and included YRab7 and YRab39. YRab35 resembled both class II and class III, as it accumulated apically in all tracheal cells (d), whereas it localised to puncta near Stac vesicles in FCs (b). (a) Confocal images of living embryos expressing the indicated endogenously YFP-tagged Rab protein (green in merge) and the membrane marker palmitoylated mCherry (palm-mCherry; magenta) in all tracheal cells. Tracheal DB FCs are shown. YRab1 (Golgi apparatus) is present in large punctate structures. YRab4 (early/recycling endosomes) is distributed in puncta of variable size. YRab5 (early endosomes) is distributed in puncta along the basolateral membrane close to the FC-FC interface. YRab7 (late endosomes and multivesicular bodies) is present on large puncta marked also by palm-mCherry, and is polarised towards the cytoskeletal track region in FCs. YRab10 (trans-Golgi network) shows diffuse signal and is distributed in small puncta throughout the cells. YRab11 (recycling endosomes) and YRab23 (polarised trafficking) are enriched on apical membranes. YRab18 (lipid droplets) is distributed in puncta enriched on the basolateral surface. (b) Stills from time-lapse movie of DB FCs. In FCs, YRab35 (green) accumulates in large puncta (asterisks in 4.5 and 7.5 min panels) close to mCherry-StacA vesicles (magenta). Images are representative of four time-lapse movies from two experiments. (c) Live imaging of DB fusion in embryos expressing mCherry-StacA (magenta) and GFP-Rab9 (green) in all tracheal cells. Rab9 (late endosomes and recycling compartments) partially colocalises with mCherry-StacA. Here, GFP-Rab9 was overexpressed because we were unable to detect endogenously tagged YRab9 in tracheal cells, although a role for Rab9 in tracheal development was previously reported27. Images are representative of seven embryos. (d) Overview of Rab proteins expressed in the embryonic tracheal system. Images are from embryos between stage 14 and stage 16. In stage 16 embryos (YRab2, YRab19, YRab39, YRabX6) the autofluorescent tracheal cuticle is visible as a bright line inside the tracheal lumen. Where not clearly visible, the luminal surface is outlined by dotted yellow lines. Results are summarized in Supplementary Table 2. Images in panels a and d are representative of three to twelve embryos. White arrowheads indicate FC-FC interface. Red asterisks indicate colocalised signals. Assignment of Rab proteins to subcellular compartments is according to ref. 25. Scale bars, 5 μm.
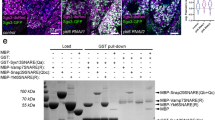
Supplementary Figure 8 Stac protein colocalises with Rab7 and Rab39.
(a–e) Confocal images from fixed and immunostained (a–c) and living (d,e) embryos expressing mCherry-StacA (magenta in merge) and an endogenously tagged Rab protein (YRab; green in merge). Each panel shows a single focal plane. Whereas YRab1 (a) signals do not overlap with mCherry-StacA signals, YRab7 (b and d) and YRab39 (c and e) show a significant degree of colocalisation with mCherry-StacA. Red asterisks above Stac vesicles in the close-up views (insets) indicate colocalisation. Note that although the fixed samples provide higher spatial resolution, the distribution of YRab7 (compare b to d) and YRab39 (compare c to e) appears more fragmented in the fixed samples compared to the living samples. Images are representative of at least 10 embryos per genotype. (f) Quantification of colocalisation between YRab proteins and mCherry-StacA in immunostained samples. The central track region of three independent FC pairs for each genotype was segmented manually. The degree of colocalisation is expressed by the ranked Kendall’s tau-b (KTB) correlation coefficient, determined in combination with Costes threshold determination. KTB values between 0 and 1 indicate colocalisation, whereas values between 0 and −1 indicate no colocalisation between the compared signals. For each condition a horizontal line indicates the KTB mean value and a vertical line indicates the standard deviation. N = 3 embryos per condition. Statistics source data are provided in Supplementary Table 3.
Supplementary Figure 9 Stac vesicles show features of Secretory Lysosomes.
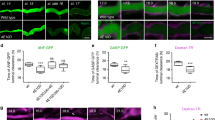
(a) Stills from time-lapse movie of DB FCs expressing GFP-Lamp1 (green) and mCherry-StacA (magenta). mCherry-StacA signals colocalise partially with Lamp1-GFP. Images are representative of six embryos and two experiments. (b) Live imaging of stage 16 embryos expressing either GFP-Lamp1 (green, upper panels) or EGFP-StacA (green, lower panels) in all tracheal cells. Embryos were injected with Lysotracker-TexasRed (magenta) to label acidic organelles. Most of the GFP-Lamp1-marked structures in FCs contain Lysotracker, with some structures showing low-intensity signals (red asterisks). EGFP-StacA compartments show either no (red asterisk on right FC) or weak Lysotracker staining (red asterisks on left FC). Images are representative of at least 10 embryos for each condition. (c) Stills from time-lapse movie of DB FCs expressing EGFP-StacA (green) and LifeAct-Ruby (magenta) to visualize F-actin. EGFP-StacA vesicles are visible close to the LifeAct-Ruby-marked apical cortex and F-actin track, and are associated with the F-actin track at later stages (highlighted in close-up views). Images are representative of six embryos. (d) Tracheal Expression of Dynamitin (Dmn) or of dominant negative p150-Glued (Gl[Δ84]) cause similarly penetrant fusion defects (Dmn, N = 15 embryos, P = 0.007;Gl[Δ84], N = 15 embryos, P = 0,001; control, N = 12 embryos; Student’s t-tests and KS-tests). Cumulative bar graph shows mean values; numbers of fusion positions scored for each genotype are indicated inside bars. Fusion defects fall into three categories (panels below graph): lumen defects (partner FCs established contact, but lumen remains discontinuous), breaks (partner FCs are separated) and misguided branches (FCs of non-cognate branches engage in ectopic fusion; open arrowhead). White arrowhead indicates FC-FC interface. Frequencies of each class of defect (percent of total number of defects) are indicated to the right of each bar. Note that expression of Dmn and Gl[Δ84] leads to a high incidence of specific lumen defects (46.7 and 60% of the total number of defects, respectively), consistent with effects on membrane trafficking. However, the percentage of specific lumen defects in stac3B20 embryos is higher (75.3%), indicating that interfering with Dynein motor activity may also have more general effects, possibly impacting on the motility of tracheal cells. Statistics source data are provided in Supplementary Table 3. (e) Live imaging of DB FCs expressing EGFP-StacA alone as a control (top) or in combination with Dmn (bottom). Maximum intensity projections of 46 time points are shown. In the control, EGFP-StacA vesicles cluster along the cytoskeletal track region (dashed red lines) and near the FC-FC interface (arrowhead) in each FC. In contrast, upon Dmn expression, EGFP-StacA vesicles are not tightly clustered and EGFP-StacA puncta (encircled by white dashed lines) are found distant from the central track region. Images are representative of >10 time-lapse movies from two experiments. Scale bars, 5 μm.
Supplementary Figure 10 Model of membrane trafficking in fusion cells.
Schematic view of early (top) and late (bottom) stages of lumen fusion. Extension of the stalk cell lumen (left) into the fusion cell (right) is brought about by recycling endosomes (yellow), while, in a second step, fusion of the extending stalk cell lumen with the central lumen is mediated by Stac-positive secretory lysosomes (orange). Top: Early during fusion, Rab11-positive recycling endosomes (a, yellow) contribute to expansion of the two apical domains (magenta), which are possibly being pulled towards each other by the cytoskeletal track (dark green). Arl3-mediated maturation of late endosomes (b, dark red) gives rise to secretory lysosome-like vesicles (orange), which are marked by Rab39, Lamp1 and Stac, and are brought into vicinity of the apical membrane domains through Dynein-mediated anchoring to microtubules. Bottom: Late during fusion, Stac recruits Syntaxin (red) to secretory lysosomes (c), which may engage with other SNARE proteins (blue) on opposite membranes. The local release of Ca2+ (d) from ER close to the site of lumen fusion may trigger the formation of mature SNARE complexes between the juxtaposed Stac vesicle and apical plasma membrane to promote fusion (e). The stage after completion of fusion, where the intracellular FC lumen has expanded, is depicted as a small cartoon at the bottom.
Supplementary information
Supplementary Information
Supplementary Information (PDF 4335 kb)
Staccato vesicles form only in tracheal fusion cells.
Time-lapse movie of an embryo expressing EGFP-StacA in all tracheal cells under the control of btl-Gal4. Partner DBs were imaged during fusion. EGFP-StacA is distributed throughout the cytoplasm of tracheal cells, whereas in FCs (indicated in the first frame) it accumulates on dynamic membrane compartments, which move along the future axis of lumen formation after partner FCs have established contact. Before contact formation, EGFP-StacA compartments are transiently found also in filopodia and cell protrusions (an example is indicated by an arrow at 19–39 min). Stills of selected time points from the second FC pair from the bottom are shown in Fig. 2c. A frame was acquired every 90 s. (AVI 5555 kb)
Staccato vesicles move in the cytoskeletal track region in a dynein-dependent manner.
Time-lapse movie of fusing DB branches of embryos expressing EGFP-StacA in all tracheal cells under the control of btl-Gal4. EGFP-StacA is expressed alone in the control (first part of movie) or in combination with the dynein regulatory subunit Dynamitin (Dmn; second part of movie). Dmn expression leads to impaired dynein-mediated transport. Upon Dmn expression, movement of Stac vesicles appears disorganised compared to the control situation. Stac vesicles scatter throughout the cells and their movement seems not to be constrained to the central track region, suggesting that anchoring of the vesicles to microtubules is lost. The movie refers to Supplementary Fig. 6e. (AVI 2220 kb)
Localised calcium transients correlate with tracheal lumen fusion.
Time-lapse movie of DB1 (upper) and DB2 (lower) branches of an embryo expressing GCaMP5 (green) and mCherry-NLS (magenta) in all tracheal cells. Two types of Ca2+ dynamics are observable in tracheal cells. In whole-cell Ca2+ spikes, the Ca2+ concentration rapidly increases throughout the entire cytoplasm (visible in the tip of DB2 at minutes 4, 8, 11, 15 and 40, and in DB3 at the end of the movie). In localised Ca2+ sparks, the Ca2+ concentration rapidly increases in small regions of the FCs. Arrows indicate several such events. Localised Ca2+ sparks can be observed in all FCs. Their duration is much shorter than that of whole-cell spikes, and they occur predominantly in the vicinity of the fusing luminal membranes. The movie refers to Fig. 7b and c. A frame was acquired every 3 s. (MOV 43125 kb)
ER exit sites are polarised towards the cytoskeletal track region of fusion cells.
Time-lapse movie of DB1 (upper) and DB2 (lower) branches of an embryo expressing myr-GCaMP5 (green) and the ER-exit site marker mCherry-Sec31 (magenta; shown as single channel on the right) in all tracheal cells. ER-exit sites are uniformly distributed throughout the cell body of tracheal stalk cells, whereas in FCs ERES are polarised towards the central track region and the FC-FC interface (indicated by arrows in all frames) during fusion. The movie refers to Fig. 7d. A frame was acquired every 3 s. (MOV 34879 kb)
Calcium levels on fusion cell apical membranes increase during lumen fusion.
Time-lapse movie of DB1 branch fusion of an embryo expressing myr-GCaMP5 (green; shown as single channel in the lower panel) and myr-tdTomato (magenta) in all tracheal cells. myr-GCaMP5 and myr-tdTomato are visible along the entire tracheal cell membrane, although myr-GCaMP5 signals are elevated apically. myr-GCaMP5 signals gradually increase on FC apical membranes, compared to the apical domains of neighboring cells. myr-GCaMP5 signal is most intense at the FC–FC interface (indicated by arrows in all frames) at the time of membrane fusion. The movie refers to Fig. 8c–f. A frame was acquired every 3 s. (AVI 30678 kb)
Rights and permissions
About this article
Cite this article
Caviglia, S., Brankatschk, M., Fischer, E. et al. Staccato/Unc-13-4 controls secretory lysosome-mediated lumen fusion during epithelial tube anastomosis. Nat Cell Biol 18, 727–739 (2016). https://doi.org/10.1038/ncb3374
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3374
This article is cited by
-
Integrin α3β1 promotes vessel formation of glioblastoma-associated endothelial cells through calcium-mediated macropinocytosis and lysosomal exocytosis
Nature Communications (2022)
-
Mapping the molecular steps of secretory-lysosome-driven tracheal tube fusion
Nature Cell Biology (2016)